Katie McLellan
Doctor of Philosophy, Graduate Program in Rehabilitation Science
Loma Linda University, June 2008
Dr. Jerrold Petrofsky, Chairperson
Preliminary research indicates there is some impairment of the endothelial cells to respond to single stressors such as local heat, shear stress, and global heat in older people and people who have diabetes. The response of the skin blood flow to local heat is an indicator of the endothelial cells ability to cope with stress. In addition, factors such as thicker subcutaneous fat and skin moisture may impair the skin’s ability to dissipate heat. For older people and people with diabetes, this endothelial dysfunction may cause an even more diminished response to multiple stressors. However, no studies have examined the effects of multiple stressors on the response of the endothelial cell in these same populations. Therefore, the purpose of this research was to build on previous studies by looking at multiple stressors, including heat dissipation, local pressure, global heat, and local heat, applied simultaneously. In these studies, older non-diabetic subjects (mean age 64.2±14.0 yrs, n=15), subjects with diabetes (mean age 62±5.9 yrs, mean duration 13.2±9.1 yrs, n=15), and younger non-diabetic subjects (mean age 25.7±2.9 yrs, n=15) participated. Subjects were exposed to three temperatures of global heat: 16°, 24°, and 32°C. The first study examined the effects of global heat and local heat with a set calorie heat load on the ability of the skin to dissipate the heat. The results indicated an inability of the endothelial cells to respond to multiple stressors in older people and xii people with diabetes. From this study, two subsequent studies were designed to look at the effects of multiple stressors on the response of the endothelial cells. First, the effects and interaction of local heat and global heat were examined. We found the response of the endothelial cells in older people and people with diabetes reached a plateau after a combined stress was placed on them. Secondly, the effects of another type of endothelial stressor, shear stress, were examined by applying different pressures to the skin and measuring the pressure-induced vasodilation (PIV). The results showed a reduction in the PIV response of the subjects with diabetes in all global temperatures indicating an inability to respond to both pressure and global heat stressors.
Thus, endothelial dysfunction, such as is commonly found in older people and people with diabetes, inhibits the endothelial cells from responding to multiple endothelial stressors. In a clinical setting, extra precautions must be taken with any type of thermal or compressive therapies done on patients with or at risk for endothelial dysfunction.
Chapter One
Introduction
Evidence from this laboratory has shown that when local heat is applied to the skin via a set caloric load (ex. heat core), older people and people with diabetes had a diminished blood flow response and ability to dissipate the heat from the skin (Petrofsky et al. 2006). Heat dissipation in the skin is brought about through convective heat transfer into surrounding tissues and through increases in blood flow (Pennes 1948). When local heat is applied to the skin, the skin blood flow increase is biphasic (Charkodian 2003, Kellogg 2006). The initial increase in blood flow involves an axonaxon reflex mechanism in which the adrenergic vasoconstrictor system releases the vasoconstrictor tone (Pergola et al. 1993, Minson et al. 2001), however, there is a secondary sustained increase in skin blood flow which is mediated by an increased release of nitric oxide by the endothelial cells (Minson et al. 2001, Kellogg et al. 1995). People who are older and who have diabetes commonly have endothelial dysfunction (Petrofsky et al. 2008, Schalkwijk and Stehouwer 2005). This may cause their response to endothelial stressors to be diminished or absent.
The previous heat dissipation studies were done at thermally neutral global temperature. Studies show there is impairment of the function of the endothelium in different global temperatures (Ho et al. 1997, Sagawa et al. 1988). Global temperature changes act as a stressor on vascular endothelial cells (McLellan et al. 2008). When an older person with or without diabetes is exposed to a warm global temperature, skin blood flow in the extremities is significantly lower than that of a younger, healthier person (Ho et al. 1997, Sagawa et al. 1988). In a previous publication, with multiple stressors, the autonomic nervous system was unable to cope in people with diabetes (Petrofsky et al. 2006). These factors together may account for a reduced response of skin blood flow to global or local heat as indicated by increased skin temperatures and reduced skin blood flow.
Traditionally, skin temperatures can be predicted using the Pennes Model which helps explain and calculate limb temperatures in response to heat (Pennes 1948). This series of equations was intended to predict the temperature in resting skin and deeper tissues. Few studies have been done to further expand upon Pennes’ equations to predict skin temperature in the diabetic and older populations. The Pennes model did not take into consideration skin moisture and subcutaneous fat, both of which may alter conductive heat exchange (Petrofsky et al. 2008, Ardevol et al. 1998). When compared to age-matched non-diabetic people, those with type II diabetes have thinner skin (Petrofsky et al. 2006, 2007, 2008, Menhert et al. 2002), thicker subcutaneous fat (Petrofsky et al. 2008), less skin moisture (Siddappa 2003, Roberts et al. 1977), and reduced skin blood flow due to greater endothelial dysfunction (Pergola et al. 1993, Minson et al. 2001, Houghton et al. 2006, Holowatz et al. 2002, Stransberry 1999, Holowatz et al. 2002, Charkoudian 2003). Based on the changes in subcutaneous fat thickness, skin moisture, and circulation with aging and diabetes, there is reason to believe that the Pennes model will not be correct in predicting skin temperatures when these variations are added to the existing model. In addition, there is some impairment with local heat and impairment with global heat, however, when the two stressors are 3 combined, it is not known how older people and people with diabetes with endothelial dysfunction will respond.
Endothelial dysfunction can also alter the response of the endothelial cells to nonthermal stressors such as shear stress through local pressure. Local pressure causes a temporary increase in blood flow, known as a pressure-induced vasodilation (PIV), which causes a subsequent release of NO that causes vasodilation (Garry et al. 2005). PIV is a natural protective mechanism that temporarily increases skin blood flow to keep the tissues from becoming hypoxic (Fromy et al. 2000, 2002). Factors such as age and diabetes can reduce the protective response of PIV. Therefore, a more comprehensive study needs to be done to examine the PIV of subjects with and without diabetes with multiple endothelial stressors.
To examine the response of vascular endothelial cells to multiple stressors, such as local heat, global heat and pressure, a series of experiments was conducted. The first study examined the ability of the skin to dissipate heat in various global temperatures, as well as the influence of subcutaneous fat thickness and skin moisture levels on the heat dissipation properties of the skin. The results pointed to an inability of the endothelial cells to respond to multiple stressors. To further investigate these findings, the second study was designed to look at the effects of local heat and global heat as endothelial stressors and the interaction of the two on skin blood flow. Based on the findings of the second study, the intent was to expand upon previous research by examining the reactive hyperemia following local pressure. The purpose was also to investigate the effects of global temperature on endothelial dysfunction by repeating the series in a thermally neutral, cool, and warm climate to examine the effects of resting blood flow on PIV for subjects who were older and who had diabetes.
Chapter Two
The Effects of Skin Moisture and Subcutaneous Fat Thickness on the Ability of the Skin to Dissapate Heat in Young and Old Subjects, With and Without Diabetes, at Three Environmental Room Temperatures
Abstract
Background: The Pennes model predicts the ability of the skin to dissipate heat as a function of conductive heat transfer and blood flow. Conductive heat exchange may be affected by skin moisture and subcutaneous fat thickness, factors not considered by Pennes. In the present investigation, we sought to expand the Pennes model by examining subcutaneous fat and skin moisture as factors of heat dissipation and their effects on heat exchange.
Methods: Subjects who were older and without diabetes (O) (mean age 64.2±14.0 yrs, n=15), had diabetes (D) (mean age 62±5.2 yrs, mean duration 13.2±9.1 yrs, n=15), and were younger without diabetes (Y) (mean age 25.7±2.9 yrs, n=15) participated. Thermisters were placed in an iron heat probe and on the skin to measure the change in skin temperature to create a thermal index to demonstrate the ability of the skin to dissipate heat.
Results: The lower back had the thickest subcutaneous fat layer for all subjects, which contributed to higher skin temperatures in response to local and global heat than the foot and hand. There was a significant inverse correlation between skin moisture and skin temperature after 5 seconds of heat application (r=-0.73, p<0.001), with O and D having significantly less skin moisture than Y (p<0.05). O and D had significantly increased skin temperatures in response to local heat as compared to Y in all global temperatures (p<0.05).
Conclusions: The Pennes model may need to be adjusted to take into consideration aging, diabetes, skin moisture, and subcutaneous fat thickness as factors of heat dissipation.
Background
The Pennes model predicts there are two basic ways the skin dissipates heat: blood flow and conductive heat loss (Pennes 1948), which is a function of skin moisture (Petrofsky et al. 2008a). The Pennes model did not take into consideration skin moisture and subcutaneous fat, both of which may alter conductive heat exchange (Petrofsky et al. 2008a, 2008b, Ardevol et al. 1998). In addition, aging and diabetes alter skin blood flow (Petrofsky et al. 2005a, 2006a, 2007a, 2008c) and may alter subcutaneous fat thickness and skin moisture.
Subcutaneous fat and its ability to alter heat transfer were not taken into consideration by the Pennes model. Subcutaneous fat thickness has been shown to vary in people who are younger, older, and who have diabetes (Petrofsky et al. 2008c, 2008d). Preliminary data in this laboratory shows transfer to be altered in people with thicker subcutaneous fat layers because there is a change in the time constant which delays heat dissipation into deeper layers (Petrofsky 2008a). Subcutaneous fat also impairs the ability of circulation to transfer heat into deeper tissues (Petrofsky and Lind 1975a, 1975b) which may contribute to higher skin temperatures during both local and global heating (Petrofsky et al. 2008e, Webb 1992).
Previous studies indicate skin moisture levels also vary the ability of heat to transfer through skin (Maglinger et al. 2005), as well as the microcirculation of the skin (Petrofsky et al. 2008a). Water has a high thermal index of 1.0, and conversely, iron has a low thermal index of 0.095 which means one calorie will raise 1cc of water 1°C, but 1 calorie raises iron almost 10°C. Skin that is dry with low water content has a lower thermal index than moist skin causing small amounts of heat to greatly increase the temperature of the skin. Studies have shown that older and diabetic populations have lower water content and drier epidermal and dermal layers (Carmeli et al. 2003, Siddappa 2003) which would indicate their skin would have a lower thermal index and be more predisposed to thermal burns. Skin moisture also affects the blood flow of the skin. A recent study from this laboratory showed a 100% increase in skin blood flow when the skin was moist versus when it was dry, as well as a reduced sensitivity for changes in blood flow with local heat applications (Petrofsky et al. 2008a). A few papers have shown that the skin may over-heat and not be able to dissipate local heat in order to avoid a burn, especially with drier skin (Shvartz et al. 1979). Therefore, a more comprehensive study needs to be made to examine the interactions of subcutaneous fat thickness and skin moisture on heat dissipation in the skin.
Older people have thicker subcutaneous fat layers and less skin moisture than younger people (Petrofsky et al. 2008a, 2008d), both of which impact the ability of the skin to dissipate heat. Older people have also been shown to have diminished blood flow responses to local and global heat (Petrofsky 2005b, 2007b, 2008e). In young and healthy people, the endothelial cells regulate the vasodilatory responses to local and global heat (Pergola et al. 1993, Minson et al. 2001, Houghton et al. 2006, Kellogg 2006, Guler et al. 2002) allowing the skin to conduct the heat into the air or through blood flow (Pennes 1948), however, older people commonly have endothelial dysfunction which impairs the microvasculature from vasodilating to dissipate the heat (Webb 1992, Carmeli et al. 2003, Ho et al. 1990, Holowatz et al. 2002).
Further compounding the problems older people have with dissipating heat is diabetes. When compared to age-matched non-diabetic people, those with type II diabetes have thinner skin (Petrofsky et al. 2006a, 2007a, 2008a, 2008d, 2008f, Menhert et al. 2002), thicker subcutaneous fat (Petrofsky et al. 2006a, 2007a, 2008a, 2008c, 2008d, 2008f), less skin moisture (Siddappa 2003, Roberts et al. 1977), and reduced skin blood flow due to greater endothelial dysfunction (Pergola et al. 1993, Minson et al. 2001, Houghton et al. 2006, Holowatz et al. 2002, Stransberry 1999, Holowatz et al. 2002, Charkoudian 2003). People with diabetes also have a reduced response of endothelial cells to stressors such as local heat (Minson Holowatz et al. 2002, 2001, Houghton et al. 2006, Johnson et al. 1986), global heat (Petrofsky et al. 2005c), and orthostatic stress (Petrofsky 2007b, Rowell 1983, 1986).
Based on all of the changes in subcutaneous fat thickness, skin moisture, and circulation with aging and diabetes, there is reason to believe that the Pennes model will not be correct in predicting skin temperatures when these variations are added to the existing model. Therefore, in the present investigation, we examined the effects of subcutaneous fat, skin moisture, aging, and diabetes on the thermal index of the skin in response to an application of local heat. However, to alter resting skin blood flow and skin moisture, we varied the global temperature since increased environmental temperature causes increased resting skin blood flow (Rowell 1983, Kellogg et al. 1998a, 1998b, Edholm et al. 1956, 1957) and skin moisture (Roberts et al. 1977, Fealey et al. 1989) to get a more complete picture of the interaction between resting skin blood flow and heat dissipation and to set up a database for better numerical modeling in future mathematical skin temperature prediction equations.
Materials
Subjects – A total of 45 subjects (3 groups, 15 subjects each) participated (Table 1). The subjects with diabetes must have been diagnosed with type II diabetes for at least three years, been taking the proper medication to control their blood-sugar levels, and have hemoglobin A1c levels between 6-10%. Subjects were excluded if they had any current or past heart problems, high blood pressure (>140 systolic/95 diastolic), or were taking any alpha or beta agonists or antagonists. All protocols and procedures were approved by the Institutional Review Board at Loma Linda University and subjects signed an informed consent document.
There was a significant difference in the BMI among the 3 groups (p<0.001) (Table 1.1), with the subjects with diabetes having the highest overall BMIs. There was not a significant difference between the mean ages for the older non-diabetic group and diabetic group (p=0.58). In terms of the Ankle-Brachii Index (ABI), none of the 45 subjects had an ABI score lower than 0.95. The subjects with diabetes had a mean hemoglobin A1c of 7.6 ± 1.1% and had been diagnosed for a mean of 13.2 ± 6.1 years.
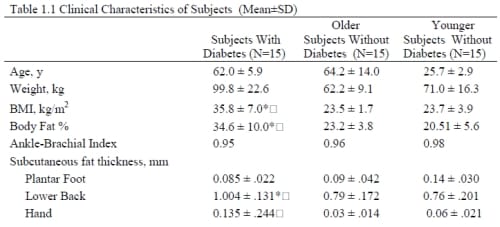
Table 1.1 Clinical Characteristics of Subjects (Mean±SD)
Methods – Control of Environmental Temperature
Environmental temperature was controlled using a controlled environment room. The room has thermal homogeneity (laminar flow) with air flow maintained with independent heating and cooling systems capable of controlling room temperature over a range from 0° to 55°C. The application of two independent heating and cooling systems allows rapid changes in room temperature, up to 7°C/minute, and the environmental temperature to be accurately controlled to within +/- 0.5°C. Three room temperatures were used: 16°, 24°, and 32°C. Relative humidity was maintained at 35 ± 10%.
Measurement of Heat Dissipation
The ability of the skin to dissipate heat was measured by applying a 90 gram iron cylinder (probe) to the surface of the skin (diameter 25mm). The sides and top of the cylinder were coated with polyurethane foam to avoid heat loss. A small hole was drilled (1mm) through the side of the iron probe and a thermistor was inserted into the center of the probe to measure the temperature of the iron probe. The hole was sealed with metal epoxy. The iron probe was held in a thin plastic bag and placed in a water bath until it was raised to the target temperature (39.5°C). The probe was then removed from the plastic bag in the water bath and placed on the skin. A second thermistor was placed on the skin directly under the probe. The change in probe versus skin temperature was assessed over a 30 second period of time to measure the heat transfer from the probe to the skin. The data from the thermistor in the probe was conditioned and amplified by a SKT100C Biopac module and digitized with a 16-bit analogue to digital converter and a Biopac MP 100 system at 2000Hz. Before each experiment, the thermistors were calibrated in a controlled temperature water bath against a standard thermometer to assure accuracy in the temperature measurements. Heat dissipation was measured at three skin sites: the back of the hand between the 2nd and 3rd metacarpal, the lower back 7cm lateral of the L3-L4 vertebrae, and over the head of the 1st metatarsal on the ball of the foot.
Measurement of Skin Temperature
A Biopac MP 100 laboratory system was used to monitor and record skin temperature. A small thermistor was placed on the skin under the Pettier junction to constantly monitor the temperature of the skin. The output of the temperature sensors was transduced by a temperature transducer module (SKT100C, Biopac Inc., Goleta, CA) and the data recorded was digitized with a 16 bits A/D Converter at 200 samples/second on a Biopac MP 100 system (SKT100C Biopac Inc., Goleta, CA). The software used was Acknowledge version 3.9.1. The temperature transducers were calibrated at the beginning of the study and periodically during the experiment.
Measurement of Skin Moisture
A Quantitative-Sweat (Q-Sweat) Measurement System was used to measure skin moisture (WR Medical Electronics Co., Stillwater, MN). Regional sweat rates were measured on the ball of the foot, lower back, and the back of the hand by applying a capsule to the skin with Velcro straps so as to not occlude skin blood flow. The crosssectional area of the capsule was 1 cm2. Airflow was directed through the inside of the capsule where it mixes with the air against the skin and returns to the measurement system via a second tube leaving the capsule. The airflow going into the capsule was measured with flow meters. Humidity sensors measured the moisture content of the returning air. The airflow and the temperature of the air going into the capsule were regulated. Sweat rate was calculated by using the change in humidity across the capsule, the airflow, and air temperature using standard tables. Skin moisture was measured for 15 minutes. The Q-Sweat capsules were placed in the same three sites as where heat dissipation was measured.
Measurement of Galvanic Skin Resistance (GSR)
Two electrodes (EL507, Biopac Inc., Goleta, CA) were placed on the skin and connected to a galvanic skin response amplifier (GSR100B, Biopac Inc., Goleta, CA). The amplifier measured the electrical resistance of the skin by passing a weak current (0.5 volts) through the skin and measuring the resistance in electricity flow between the electrodes. To measure GSR, the skin was debrided with an abrasive pad, cleaned with an alcohol swab, and then the electrodes sat on the skin for 10 minutes before any measurements were taken. The electrodes were placed on the skin 5 cm apart and measurements were recorded for 2 minutes. The mid-point between the electrodes was the center of where the Q-Sweat capsule had been placed. While GSR was being measured, all noises and sounds in the room were silenced and the patient was asked to remain quiet so that no external stress, arousal, or emotional excitement would influence or interfere with the measurements. GSR assesses the water content of the skin by measuring the electrical resistance of the epidermal and dermal layers.
Measurement of Subcutaneous Fat Thickness
A SonoSite 180 Plus ultrasound system (SonoSite, Inc., Bothell, WA) was used to measure the subcutaneous fat thicknesses of the ball of the foot, lower back, and the back of the hand. The device provides a weak ultrasound signal at a frequency of 10 million cycles per second. The ultrasound signal reflected from the muscles below the probe creates a two-dimensional picture to measure the thickness of the skin, fat under the skin, and underlying muscle. A 38 mm broadband (10-5 MHz) linear array transducer for musculoskeletal imaging was used. The skin and ultrasound transducer were coated with a water-based ultrasound gel and a 1cm stand-off was used.
Measurement of Ankle-Brachial Index (ABI)
Blood pressure measurements were taken at the ankle and brachial artery in the arm using a standard sphygmomanometer cuff and stethoscope. The ankle and arm systolic blood pressure measurements for the left and right limbs were recorded. Then, the ankle systolic pressures were divided by the highest arm pressure to establish the ABI for each leg. The ABI range that is generally considered normal is 0.95 to 1.2.
Measurement of Total Body Fat Percent
The total body fat percent was measured by an impedance plethysmograph (RJL systems, Clinton Twp., MI). The Quantum II provides high-resolution whole body and regional bioelectrical resistance and reactance measurements. The total body fat percent was measured through electrodes placed on the right foot and right hand.
Procedures
To establish the inter-relationships between subcutaneous fat thickness, skin moisture, aging, and diabetes, a series of experiments was conducted whereby the subjects sat in a controlled environment room (16°, 24°, and 32°C) on different days for 20 minutes to acclimate. A heat core was applied to the skin at 39.5°C for 30 seconds and the effects of subcutaneous fat, skin moisture, aging, and diabetes were measured. Since the resting blood flow in the skin can alter heat dissipation, according to the Pennes model, the baseline blood flow was at three different levels due to the changes in global temperature. This was done to further examine the effects of skin moisture, aging, and diabetes on heat dissipation.
At the start of the study, ultrasound was done on all of the subjects to measure the thickness of the subcutaneous fat on the ball of the foot, lower back, and the hand prior to the subject acclimating in the temperature-regulated room. Once the subjects had acclimated, baseline skin temperatures were taken and the skin moisture was measured with GSR and the Q-Sweat system.
Data Analysis
In order to compare the ability of the skin to dissipate heat, a thermal index as formulated to measured the change in skin temperature over a period of 5 seconds. Calculations of means and standard deviations (SD) were done and all values are expressed as mean ± SD. Comparisons among groups were performed using one-way ANOVA, independent t-tests, Pearson correlation, and linear regression. The level of significance was p=0.05. The results were analyzed using SPSS version 15.0.
Results – Heat Dissipation
Of the 30 seconds the probe was placed on the skin, the greatest change in skin temperature occurred in the first 5 seconds of application. The hand and foot responded similarly in all three global temperatures and typical results are shown for the foot (Figure 1.1). The younger subjects had no significant differences in the thermal index of the lower back in any of the global temperatures (p=0.08), but there was a significant difference for the foot (p<0.001).
Thermal Index of the Foot and Lower Back
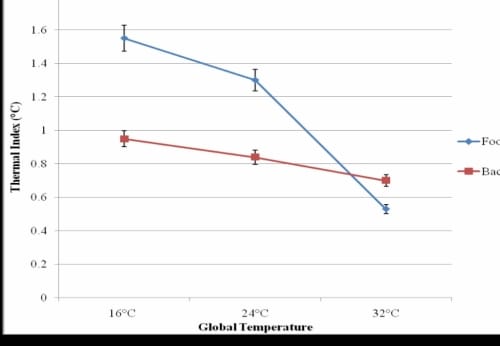
Figure 1.1 – This figure illustrates the thermal Index of the foot and lower back of younger non-diabetic subjects with standard deviation error bars.
Effects of Aging
There was a significant correlation between age and thermal index of the foot in all three global temperatures (r16°C = 0.53, p16°C < 0.001; r24°C = 0.67, p24°C < 0.001; r32°C=0.75, p32°C<0.001), but not for the lower back. There was a significant difference in the thermal index of the younger and older non diabetic subjects in the three global temperatures (p16°C<0.0001, p24°C<0.0001, p32°C<0.0001) (Figure 1.2).
Thermal Index of the Foot
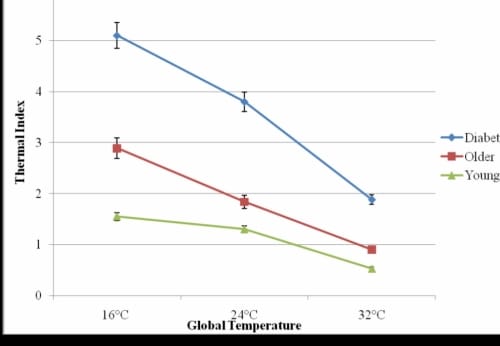
Figure 1.2. This figure illustrates the thermal index of the foot- the change in skin temperature over 5 seconds with standard deviation error bars.
In the 16°C global temperature, the younger and older without diabetes subjects had an increased skin temperature mean of 1.55±0.3°C and 2.89±0.5°C, respectively, during the first 5 seconds the heated probe was placed on the foot. In the 32°C global temperature, the difference in thermal indexes was reduced with a mean increase of 0.9±0.005°C for the older subjects and 0.53±0.002°C for the younger subjects on the foot. On the lower back, the results were similar for the older subjects, however, for the younger subjects, the thermal index was 38% and 55% higher in the 16°C and 24°C global temperatures and 32% lower in the 32°C global temperatures.
Effects of Diabetes
The subjects with diabetes had the highest thermal indexes in all three global temperatures, and typical results are shown for the foot (Figure 1.2). The temperature on the skin of the foot increased significantly more than for the subjects with diabetes than the older subjects without diabetes (p<0.05) and younger subjects (p<0.001) in the 16°, 24°, and 32°C global temperatures. In the 16°C global temperature, the subjects with diabetes had a thermal index of 5.1±0.6°C which was 176% and 329% higher than the thermal index for the older and younger subjects without diabetes, respectively.
The subjects with diabetes had a significantly higher thermal index of the foot than the older and younger subjects in all global temperatures (p16°, 24°C <0.001, p32°C =0.03). A forward linear regression test showed the presence of diabetes accounted for 88.1% of the unexplained variability in the thermal index of the foot in the 16°C global temperature with age being excluded. In the 24°C and 32°C global temperatures, age and diabetes accounted for 97.1% and 94.5% of the unexplained variability in the thermal index, respectively.
Effects of Skin Moisture
There was a significant inverse correlation between skin moisture and age in the 16°C global temperature (r=-0.65, p<0.001), the 24°C (r=-0.69, p<0.001), and the 32°C global temperature (r=-0.80, p<0.001) (Figure 1.3).
Correlation between Age and Skin Moisture
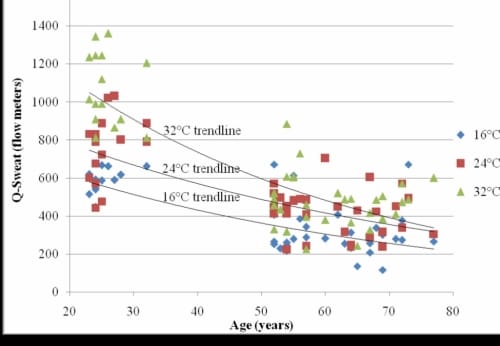
Figure 1.3. This figure illustrates the correlation between age and skin moisture as measured by the Q-Sweat system in three global temperatures with exponential trendlines.
There was a significant inverse correlation between Q-Sweat and GSR (r=-0.89, p<0.001) and typical results for each global temperature are shown for the Q-Sweat measurements (Figure 1.4). The subjects with diabetes had a significant decrease in skin moisture of the foot as measured by the Q-Sweat system (p=0.01) and a significant increase in GSR (p=0.03) in all three global temperatures compared to the older subjects without diabetes. There was also a significant inverse correlation between skin moisture and thermal index (r=-0.73, p<0.001) (Figure 1.5).
Q-Sweat Skin Moisture Measurements
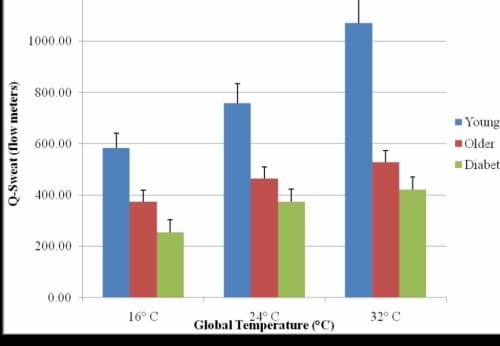
Figure 1.4. This figure illustrates the Q-Sweat skin moisture measurements of the foot in three global temperatures with standard deviation error bars.
Correlation between Skin Moisture and Thermal Index
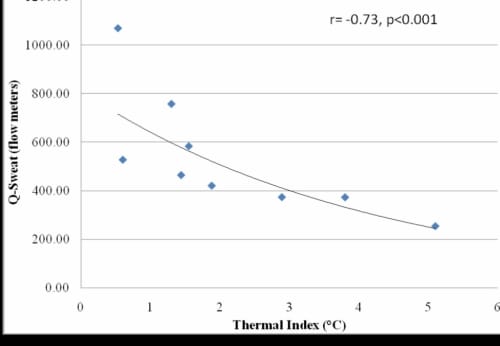
Figure 1.5. This figure illustrates the correlation between skin moisture and thermal index with exponential trendline.
Effects of Subcutaneous Fat Thicknesses
There was not a significant correlation between subcutaneous fat thickness of the foot and thermal index in any of the global temperatures (r16°=0.08, p16°=0.59; r24°=0.03, p24°=0.84; r32°=-0.01, p32°=0.97).
A forward linear regression with age, presence of diabetes, skin moisture, and subcutaneous fat thickness of the foot excluded subcutaneous fat thickness from the model for predicting thermal index. These three independent variables accounted for 97.4% of the unexplained variability of the thermal index.
There was a significant difference between the subcutaneous fat thickness of the foot and lower back for all subjects (p=0.02) (Figure 1.6). The increased subcutaneous fat thickness on the back contributed to both positive and negative percent differences in the thermal index of the foot as compared to the lower back. All subjects had a higher thermal index for the back than for the foot in the 16°C and 24°C global temperatures.
Subcutaneous Fat Thickness of the Foot and Lower Back
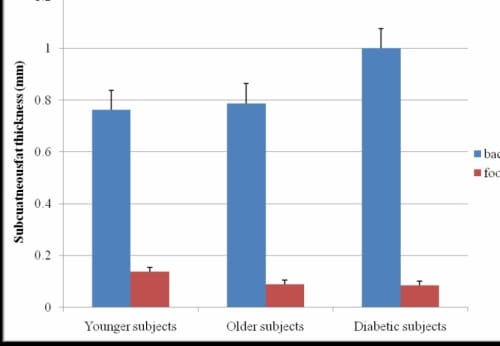
Figure 1.6 This figure illustrates the subcutaneous fat thicknesses of the foot and lower back with standard deviation error bars.
Discussion
The classical “bioheat” approach of Pennes says the heat flow to or from the tissues is proportional to the product of perfusion rate and the difference of arterial and tissue temperature (Brink and Werner 1994). This series of equations was intended to predict the temperature in resting skin and deeper tissues. The following assumptions were stated to help predict heat transfer: 1) the cross-section of the arm is almost a perfect cylinder, 2) the tissue of the forearm contains two heat sources (tissue metabolism and heat transferred from blood to tissue at each point in the forearm), and 3) the flow of blood per unit column of tissue per second is considered uniform throughout the forearm (Pennes 1948). To make these assumptions, Pennes relied on data from studies on animal tissues, used values in his equation that do not correspond to values found with human skin, failed to normalize data, and did not use corresponding locations on all subjects (Wissler 1985). Pennes also assumed that the predominant site of heat exchange in the skin is through the capillary bed. Analytical data has shown heat may be exchanged in the blood before it reaches the arterioles (Chato 1980, Chen et al. 1980). Pennes’ approach may deliver acceptable results if the body is in the thermoneutral zone or if heat stress acts uniformly on the whole body, however, since 1948, these assumptions have been refuted or criticized by more recent physiological studies (Wissler 1985, Nelson 1998).
Data from this present study seeks to further expand the numerical modeling that is needed to accurately apply the Pennes’ “bioheat” equation to a wider range of conditions and populations. Current data indicates age, the presence of diabetes, moisture levels of the skin, thickness of subcutaneous fat, and global temperature affect the amount the skin heats up in the first 5 seconds of a local heat application. Younger subjects were shown to have the smallest thermal index in all three global temperatures. The subjects who were older without diabetes had higher skin temperatures in response to local and global heat, drier skin as indicated by Q-Sweat and GSR measurements, and thicker subcutaneous fat on the lower back and hand. There was also a significant inverse correlation between age and skin moisture in the three global temperatures. Together, these factors may help account for the higher incidence of burns and heatrelated illnesses in older people (Pandolf 1991, Bessey et al. 2006, Brigham and McLoughlin 1996). When older people with diabetes were compared with older subjects without diabetes, the presence of diabetes resulted in significantly higher thermal indexes in all global temperatures, significantly drier skin as measured by the Q-Sweat system and GSR, and thicker subcutaneous fat on the lower back.
In this study, we used changes in global temperature to alter resting skin blood flow to see how various amounts of resting blood flow affected heat transfer. With short exposure times to local heat, the vasculature of the skin may rely on heat shock proteins (HSP), which act as chaperones to the vascular endothelial cells in regulating NO levels and to promote NO generation and vasodilation (Sud et al. 2007, Ou et al. 2003). Animal models have shown HSP90 to promote NO generation through endothelial nitric oxide synthetase (eNOS) activity and vascular reactivity (Sud et al. 2007, Aschner et al. 2007). High levels of NO in endothelial cells activate quanylate cyclase (CG) production (Bellien et al. 2008, Benabdallah et al. 2008, Comerford et al. 2008, Medeiros et al. 2008) which facilitates the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) that allows the smooth muscle surrounding blood vessels to dilate as potassium permeability is increased (Benabdallah et al. 2008). The prevalent inability to dissipate heat in older and diabetic populations may be due to a number of factors. HSP90 activity may be reduced with endothelial dysfunction commonly found in people who are older and with diabetes (Sud et al. 2007, Swiecki et al. 2003, Huang 2003, Lei et al. 2007), there could be a dysfunction of TRP channels required to synthesize TRPV4 which increase calcium influx to vasodilate the skin (Kwan et al. 2007).
In addition, the observed increases in the thermal index of the lower back versus the foot may be due to a thicker subcutaneous fat layer which has recently been shown to insulate the skin from dissipating the heat into deeper tissues (Petrofsky et al. 2008a, 2007b). Recent studies examined the effects of long-term heat exposure on similar groups of subjects; younger, older, and with diabetes (Petrofsky et al. 2007a, 2008a). With age, there is a reduction of red blood cell concentration in the skin at rest and after local heat possibly due to fewer blood vessels in the skin and a thinning of the dermal layer (Petrofsky et al. 2008f). With low circulation in the skin, the temperature of the skin can increase as heat accumulates and is not dissipated into the blood or deeper tissues. The presence of diabetes resulted in further reduced skin circulation in response to contrast baths, local heating, and global heating (Petrofsky et al. 2006a, 2007a, 2007b). It is common for people who are older, with or without diabetes, to have thicker subcutaneous fat layers resulting in obesity (Petrofsky et al. 2008a, 2008b). Obesity slows dissipation of heat into deeper tissues (Petrofsky et al. 2008b, 2008f). When compounded with the presence of diabetes, the skin heats up considerably warmer and faster in response to local and global heat as compared to non-diabetic subjects who are older and younger without diabetes (Petrofsky et al. 2008b).
Conclusions
The Pennes model does well to provide a framework around which we can begin building mathematical equations to help predict skin temperatures and heat dissipation properties of the skin. The original series of equations presented in 1948 did not take into account, nor did it leave room for, the physiological differences seen in people who are older, with and without diabetes, that may have thicker subcutaneous fat layers, thinner skin, and lower skin moisture levels than the animal and human models used in the original Pennes model. As evident by the various properties of younger, older, and diabetic skin, there is a need for a more expanded database to create inclusive mathematical modeling allowing for greater number of variables in the Pennes equations.
Chapter Three
Multpile Stressors and the Response of the Vascular Endothelial Cells; The Effects of Aging and Diabetes
Abstract
Background: The present study examined the effects of local heat, global heat, and the interaction between these two endothelial stressors on the blood flow of the skin of the foot in people who are older and who have diabetes.
Methods: Subjects who were older without diabetes (O) (mean age 64.2±14.0 yrs), with diabetes (D) (mean age 62.0±5.9 yrs, mean duration 13.2±9.1 yrs), and were younger without diabetes (Y) (mean age 25.7±2.9 yrs) participated. Subjects were exposed to 3 global temperatures (16°, 24°, and 32°C) and the blood flow response was recorded on the foot with a laser Doppler flow meter for 30 seconds following the applications of local heat (30°, 33.5°, and 37°C) using a Peltier junction to clamp the skin for 2 minutes.
Results: All 3 groups significantly increased blood flow from the 16° to 24°C environments for the 37°C application of local heat (pY=0.02, pO=0.02, pD=0.01). D and O only increased blood flow 5% and 6% from the 24° to 32°C environment, which was not statistically significant (pO=0.12, pD=0.14).
Conclusions: There appears to be considerable blood flow reserve in younger subjects to tolerate heat stress. In contrast, older and diabetic subjects reach a critical level after which additional heat does not cause in increase in blood flow.
Background
Under normal resting conditions, there is vasoconstrictor and vasodilator tone in the vascular endothelial cells. Vasodilators may include nitric oxide and prostacyclin (Schiffrin et al. 2001, Verma and Anderson 2002) while prostacyclin 2 acts as a vasoconstrictor (Koch et al. 2003). Aging and diabetes commonly reduce the ability of the blood vessels to dilate leaving them predominantly vasoconstricted and unable to supply sufficient blood to the skin and organs of the body (Petrofsky et al. 2008a, 2005a, 2005b, Kellogg et al. 1998, Stransberry et al. 1999).
Local heating causes vascular endothelial cells to release vasodilators (Petrofsky et al. 2005a). The initial response to local heat involves an axon reflex mechanism in which the adrenergic vasoconstrictor system releases vasoconstrictor tone (Pergola et al. 1993, Minson et al. 2001). Neurotransmitters are also released and stimulate a small amount of nitric oxide (NO) release due to shear stress on the endothelial cells of the blood vessels (Minson et al. 2001, Houghton et al. 2006, Petrofsky et al. 2007a). The neurotransmitters released are believed to be calcitonin gene-related peptide (CGRP) and substance P, both of which are released from heat-sensitive nociceptors (Minson et al. 2001, Petrofsky et al. 2007a). After the initial vasodilator response which peaks within a few seconds or minutes, there is a brief nadir (plateau) followed by a prolonged vasodilation response that increases and maintains blood flow to a plateau (Minson et al. 2001, Charkodian 2003, Kellogg 2006, McCord et al. 2006). The secondary, sustained increase in skin blood flow is mediated by an increased release of nitric oxide by the endothelial cells (Minson et al. 2001, Kellogg et al. 1995) due to TVRP thermoreceptors (Kwan et al. 1992, Watanabe et al. 2002).
Similar to local heat, global heat causes an increase of the cutaneous vasodilation of the skin which is due to centrally mediated autonomic thermoreceptors (Kellogg et al. 1998, Petrofsky et al. 2007a). The active vasodilator system is initiated in part by a release of NO and other neurotransmitters as the temperatures of the core and skin continue to rise (Kellogg et al. 1998) causing increased blood flow to the skin by 80- 90% during heat stress (Johnson 1996, Rowell 1986).
When an older person with or without diabetes is exposed to a warm global temperature, skin blood flow in the extremities is significantly lower than that of a younger, healthier person (Ho et al. 1997, Sagawa et al. 1988). There is an attenuated vasodilatory response with advanced age (Kenney and Hodgson 1987, Weigert et al. 1995, Kenney 2008) as well as a skin thickness decrease (Petrofsky 2007a, Branchet et al. 1990, Petrofsky et al. 2008b). In addition, impaired circulation and impaired endothelial dilation, both of which are common in subjects with diabetes, may impair the increase in blood flow to the skin (Petrofsky 2007a, 2008a, Schalkwijk and Stehouwer 2005). In a previous publication, with multiple stressors, the autonomic nervous system was unable to cope in people with diabetes (Petrofsky et al. 2006). These factors together may account for a reduced response of skin blood flow to global or local heat.
According to the available literature, there is some impairment with local heat and impairment with global heat, however, when the two stressors are combined, it is unknown how older people and people with diabetes will respond. The purpose of the present study was to investigate the effects of local heat, global heat, and the interaction between these two endothelial stressors on the blood flow of the skin in people who are older and who have diabetes. We hypothesize that the blood flow response will be limited with higher amounts of combined local and global heat indicating an inability of the endothelial cells to handle additional stress.
Materials – Subjects
A total of 45 subjects (3 groups, 15 subjects each) participated. (Table 2.1) The subjects with diabetes must have been diagnosed with type II diabetes for at least three years, been taking the proper medication to control their blood-sugar levels, and have hemoglobin A1c levels between 6-10 percent. Subjects were excluded if they had any current or past heart problems, high blood pressure (>140 systolic/95 diastolic), or were taking any alpha or beta agonists or antagonists. All protocols and procedures were approved by the Institutional Review Board at Loma Linda University and subjects signed an informed consent document.
There was a significant difference in the BMIs among all 3 groups (p<0.001) (Table 1) with the diabetic subjects having the highest overall BMI’s. There was not a significant difference between the mean ages for the older non-diabetic and diabetic groups (p=0.58). In terms of the Ankle-Brachii Index (ABI), none of the 45 subjects had an ABI score lower than 0.95. The subjects with diabetic had a mean hemoglobin A1c of 7.6 ± 1.1% and had been diagnosed for a mean of 13.2 ± 6.1 years.
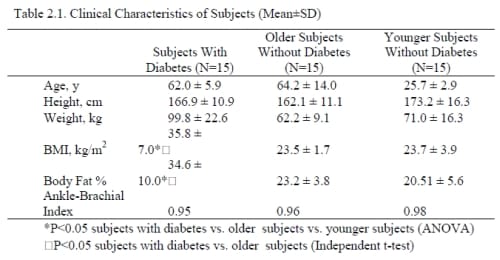
Table 2.1. Clinical Characteristics of Subjects (Mean±SD)
Methods
Application of Local Heat- A Peltier junction was used to apply local heat to the skin. The Peltier junction is a heat pump that adds or removes calories from the skin based on the flow of current through the junction. Current was applied to the Peltier junction through a Sorenson QRB 15-2 power modulator (PTB Sales, Azusa, CA) to slowly increase the temperature of the skin to 30°, 33.5° and 37° C. At each temperature, current was regulated to clamp skin at that temperature for 2 minutes prior to skin blood flow measurements. The current was measured and recorded with an HLT100C high level transducer through a Biopac MP 100 laboratory system (Biopac Inc., Goleta, CA) to establish an index for the number of calories required to heat and clamp the skin at a specific temperature. Local heat was applied and measured over the head of the 1st metatarsal on the ball of the foot.
Measurement of Blood Flow
A single point infrared laser Dopler flow meter (TST 140 probe, LDF100 module, Biopac Inc., Goleta, CA) was used to measure the skin blood flow. The skin blood flow was recorded for 30 seconds. The flow meter had a head with an active area of one square centimeter. The probe was plugged into an LDF 100C amplifier and then digitized at 2000 samples per second with a 16 bit analogue to digital converter. The converted signal was then saved for final analysis with a Biopac MP 100 system (Biopac Inc, Goleta, CA).
Control of Environmental Temperature
Environmental temperature was controlled using a controlled environment room. The room has thermal homogeneity (laminar flow) with air flow maintained with independent heating and cooling systems capable of controlling room temperature over a range from 0° to 55°C. The application of 2 independent heating and cooling systems allows rapid changes in room temperature, up to 7°C/minute, and the environmental temperature to be accurately controlled to within +/- 0.5°C. Three room temperatures were used: 16°, 24°, and 32°C. Relative humidity was maintained at 35 ± 10%.
Measurement of Skin Temperature
A Biopac MP 100 laboratory system was used to monitor and record skin temperature. A small thermister was placed on the skin under the Peltier junction to constantly monitor the temperature of the skin. The output of the temperature sensors was transduced by a temperature transducer module (SKT100C, Biopac Inc., Goleta, CA) and the data recorded was digitized with a 16 bits A/D Converter at 200 samples/second on a Biopac MP 100 system (SKT100C Biopac Inc., Goleta, CA). The software used was AcKnowledge version 3.9.1. The temperature transducers were calibrated at the beginning of the study and periodically during the experiment. The skin was heated to 30°, 33.5° and 37°C.
Measurement of Ankle-Brachial Index (ABI)
Blood pressure measurements were taken at the ankle and brachial artery in the arm using a standard sphygmomanometer cuff and stethoscope while the subject was at rest and had been lying supine for at least 5 minutes. The ankle and arm systolic blood pressure measurements for the left and right limbs were recorded. The ABI is derived by dividing the systolic pressure of the ankle by the systolic pressure of the brachial artery, and the result is used to predict the severity of peripheral arterial disease. The ABI range that is generally considered normal is 0.95 to 1.2.
Measurement of Total Body Fat Percent
The total body fat percent was measured by an impedance plethysmograph (RJL systems, Clinton Twp., MI). The Quantum II provides high-resolution whole body and regional bioelectrical resistance and reactance measurements. The total body fat percent was measured through electrodes placed on the right foot and right hand.
Procedures
On three different days, the subject sat comfortably in the controlled environment room at 16°, 24°, or 32°C for 20 minutes. Before any local heat was applied, baseline blood flow was taken. The skin was clamped at each of the three local temperatures (30°, 33.5° and 37°C) for 2 minutes. The Peltier junction was removed after the skin had been clamped for 2 minutes, the laser dopler blood flow probe was placed on the skin where the Peltier junction had been, and blood flow was again measured for 30 seconds. This was repeated in the 3 global temperatures.
Data Analysis
Calculations of means and standard deviations were done and all values are expressed as mean±SD. Comparisons among groups were performed using One-way ANOVA and independent t-tests. The level of significance was p = 0.05. The results were analyzed using SPSS version 15.
Results – Effects of Local Heat
The resting blood flows are displayed in Figure 2.1. The typical response is shown in Figure 2.2 where the global temperature was 24°C. The pattern of response was similar for all global temperatures.
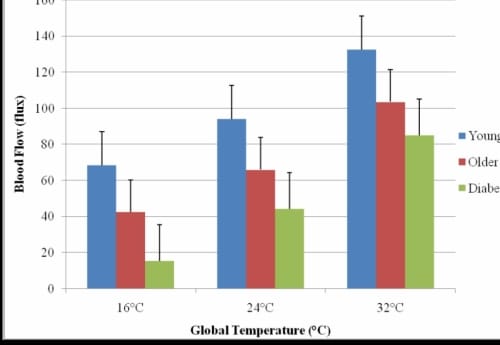
Figure 2.1. This figure illustrates the resting blood flow of the foot in each global temperature with standard deviation error bars.
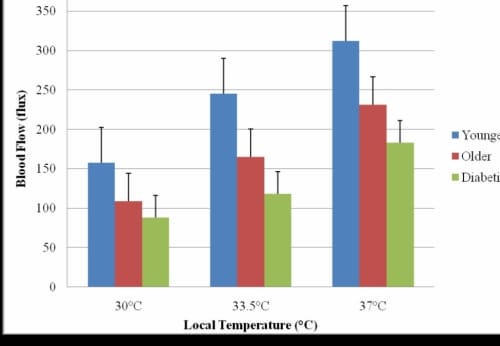
Figure 2.2. This figure illustrates the skin blood flow response after the skin had been clamped for 2 minutes at three local heat temperatures in the 24°C global temperature with standard deviation error bars.
The subjects with diabetes had a mean flow of 44.59 ± 8.04 flux which was significantly less than the mean flow of the younger subjects 94.20 ± 16.39 flux (p=0.01). When compared to the older non-diabetic subjects, the presence of diabetes resulted in 33% less skin blood flow which was significant (p=0.02).
For all three applications of local heat, the older non-diabetic subjects had significantly less blood flow than the younger subjects (p30°C=0.04, p33.5°C=0.04, p37°C=0.04). However, the subjects with diabetes had the most diminished blood flow response of all groups of subjects. The subjects with diabetes had significantly less blood flow in response to the three applications of local heat as compared to the older subjects without diabetes (p30°C<0.001, p33.5°C=0.04, p37°C<0.001). The application of 37°C heat elicited the greatest amount of change in the skin blood flow from all groups of subjects.
Skin Blood Flow Response of the Foot in Three Global Temperatures
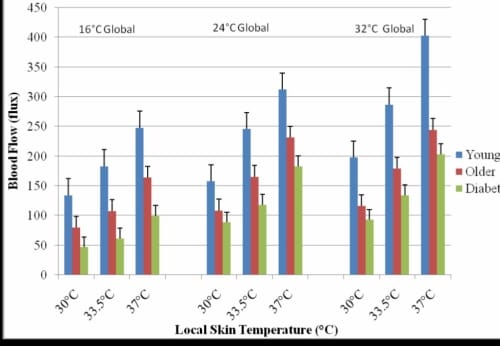
Figure 2.3. This figure illustrates the skin blood flow of the foot in response to three local heat temperatures in three global temperatures with standard deviation error bars.
The subjects with diabetes had a mean skin blood flow of 183.0 ± 31.3 flux which was 26% lower than the older non-diabetic subjects and 70% less than the younger subjects. The difference between the blood flow for the subjects with diabetes and that of the older and younger non-diabetic subjects was significant (p=0.02).
When the skin blood flow response is displayed in terms of percent increase from baseline (Figure 2.3), the younger subjects had a 348% increase in skin blood flow, the older subjects without diabetes had a 276% increase, and the subjects with diabetes only saw a 203% increase above resting. These differences in percent blood flow increase were significant (p=0.01).
Effects of Global Heat
Figure 2.3 shows the skin blood flow responses to the three local heat applications in each of the three global temperatures. The pattern of response was similar for all local temperatures and typical results are shown for 37°C. In the 16°C global temperature, the younger subjects saw a blood flow response of 247.61 ± 37.8 flux and in the 32°C global temperature this was significantly elevated to 402.50 ± 67.2 flux (p=0.01). There were no statistical differences in the blood flow change seen in the older subjects with and without diabetes. The older subjects without diabetes had an increase in blood flow from 163.89 ± 38.3 flux in the 16°C global temperature to 244.22 ± 45.1 flux in the 32°C global temperature. The subjects with diabetes had an increase from 99.35 ± 23.3 flux in the 16°C global temperature to 203.4 ± 36.8 flux in the 32°C global temperature.
The percent increase in blood flow from baseline is shown in Figure 2.4. The younger subjects had a significant increase from 261.8% above baseline in the colder room to 394.29% in the hotter room (p=0.01). The older non-diabetic subjects also had a significant increase from 177.26% in the 16°C global temperature to 294.62% in the warmer 32°C global environment (p=0.04), and the subjects with diabetes had the smallest percent change of blood flow increase from baseline with 122.63% in the 16°C global temperature and 172.9% in the 32°C global temperature. This change in blood flow was not significant for the subjects with diabetes.
Percent Blood Flow Increase from Baseline
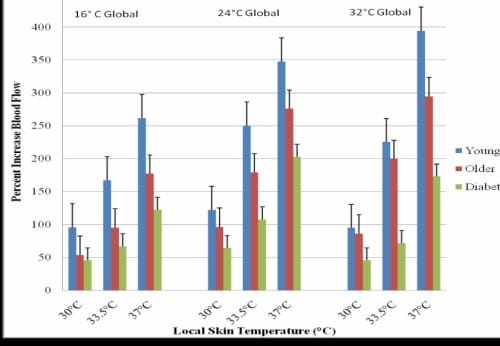
Figure 2.4. This figure illustrates the percent blood flow increase from baseline in the foot with standard deviation error bars.
Interaction of Local and Global Heat
To evaluate the interaction between local heat and global heat, the blood flow measurements for the 37°C application of local heat were used because the application of this temperature of local heat elicited the most dramatic response from the skin blood flow. Figure 2.5 displays the interaction of local heat and global heat on skin blood flow. In the cold global temperature of 16°C, there was significant difference between the skin blood flow response of the younger and older without diabetes, and diabetic subjects (p<0.001). The younger subjects had a mean blood flow of 247.61± 24.31 flux which was significantly higher than the 163.89 ± 17.8 flux recorded for the older non-diabetic subjects (p=0.04) and 99.35 ± 17.2 flux seen in the subjects with diabetes (p=0.03). The increase in blood flow from 16°C to 24°C to 32°C global temperatures was linear for the younger subjects but non-linear for the older subjects with and without diabetes.
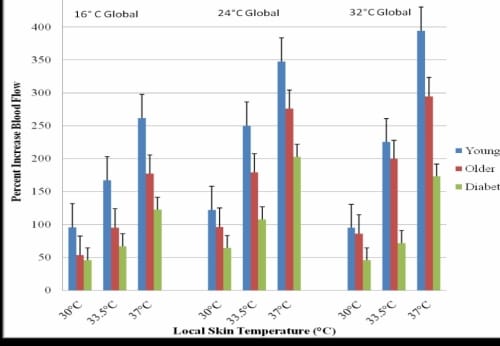
Figure 2.5. This figure illustrates the interaction of local heat (37°C) and global heat on skin blood flow of the foot with standard deviation error bars.
In the thermally neutral 24°C room, the differences were similar to differences in the colder room. There was a significant difference between the blood flows recorded for all three groups of subjects (p=0.02). The subjects with diabetes had a mean blood flow that was 26% lower than the age-matched non-diabetic subjects. The younger subjects had a mean blood flow of 312.03 ± 44.2 flux, which was significantly higher than the mean flow of 183.02 ± 13.5 flux recorded for the subjects with diabetes (p<0.001).
In the warm 32°C global temperature, the younger subjects’ mean blood flow rose to 402.5 ± 65.8 flux which was 30% above what was seen in the 24°C global temperature, however, the mean skin blood flow for the diabetic subjects and older nondiabetic subjects was not statistically different (p=0.53) and only rose 5% and 6%, respectively.
Discussion
Current research has shown that older people with and without diabetes have diminished responses of the skin blood flow when stressed with local or global heat as compared to younger non-diabetic subjects (Koch et al. 2003, Petrofsky et al. 2008a, 2005a, 2005b, Kellogg et al. 1998, Stransberry et al. 1999, Pergola et al. 1993, Charkodian 2003, Sagawa 1988). The vascular endothelial cells of older people with and without diabetes are damaged and cannot adequately induce vasodilation of the skin in response to various stimuli (Schiffrin 2001, Verma and Anderson 2002, Petrofsky et al. 2007a). When compounded with diabetes, the environment of the vascular endothelial cells is further compromised and the skin blood flow response is profoundly more diminished or even absent (Petrofsky et al. 2007b, 2008c). Local heat and global heat were used in this study to examine how endothelial cells of older people and people with diabetes respond to multiple stressors.
The current data indicates aging and diabetes results in significant decreases in the blood flow response of the skin to both local and global heat. For all three groups of subjects, there were significant increases in skin blood flow from the cooler global temperature (16°C) to the thermally neutral global temperature (24°C) due to local heat, however, in the warm global temperature (32°C), there was not a significant elevation in skin blood flow when the skin was clamped at 37°C for 2 minutes. These results may point to damaged vascular endothelial cells because the skin, in response to thermal stressors, cannot vasodilate in older and diabetic people as much as in younger people.
Older people and people with diabetes progressively have more impairment in the endothelial cells to function with combined stressors (Petrofsky et al. 2005b, 2006, 2008c). In addition, the autonomic nervous system has been shown to be damaged in older people with and without diabetes (Petrofsky et al. 2007a). If the skin of the foot is stressed by applying too much heat on the body, younger people will be able to increase their skin blood flow accordingly. Younger people appear to have a reserve in their ability to vasodilate in response to higher amounts of local and global heat. Older people and people with diabetes have blood flow that appears to plateau and only increase slightly when local heat is applied in warm environments. They may not be able to respond with increased vasodilation after the blood vessels have vasodilated to a certain size. Endothelial and autonomic dysfunction may help explain the plateau in skin blood flow response when multiple stressors are applied to the body.
Normally, when heat is applied to the skin, TRPV4 channels increase calcium (Ca+) influx exponentially above 25°C (Watanabe et al. 2002). Ca+ channel receptors activate nitric oxide (NO) synthetase to produce NO which activates guanylate cyclase (GC) production (Bellien et al. 2008, Benabdallah et al. 2008, Comerford et al. 2008, Medeiros et al. 2008). GC catalyzes the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and pyrophosphate. Once formed, cGMP increases potassium (K+) permeability allowing the blood vessels to vasodilate (Benabdallah et al. 2008).
The observed plateau in this study may be a result of a number of mechanism dysfunctions. Endothelial dysfunction is associated with malfunction and dysfunction of TRP channels required to synthesize TRPV4 (Kwan et al. 1992). The decreased activity to TRPV4 decreases Ca+ influx which reduces flow-induced vasodilation (Kwan et al. 1992). With aging and diabetes, the Ca+ channel receptors become defective causing them to be insensitive to the presence of Ca+ or the channels become blocked so Ca+ cannot get in (Tarif and Bakris 2000). Without Ca+, NO synthetase is not activated to produce NO (Bellien et al. 2008, Comerford et al. 2008). The problem may also be that, with aging and diabetes, NO synthetase cannot produce enough NO to activate GC production. More NO may be produced, but there may be a limitation on the production of GC which then limits the production of cGMP (Medeiros et al. 2008, Brock and Tonussi 2008). The cGMP system in smooth muscle may have insufficient amounts of enzymes to increase K+ permeability, thus limiting vasodilation (Medeiros et al. 2008).
The effects of endothelial and autonomic dysfunction associated with impaired vascular responses to local and global heat may help explain the high occurrence of thermal burns in older people with and without diabetes. In 1948, the Pennes “bioheat” study was published that helps explain and predict skin blood flow. The classical bioheat approach of Pennes describes that heat flow to or from the tissues is proportional to the product of perfusion rate and the difference of arterial and tissue temperature (Pennes 1948, Brink and Werner 1994, Wissler 1998). Pennes’ equation helps explain the ability of the skin to hold and transfer heat absent blood flow. The series of equations presented was intended to predict the temperature in resting skin and deeper tissues, however, few studies have been done to further expand upon the Pennes equation to predict skin temperatures in diabetic and older populations.
Conclusion
Without adequate vasodilations, in response to thermal stressors, older people, with and without diabetes, are more susceptible to burns because they cannot increase the microcirculation in the skin needed to dissipate the heat. In a clinical setting, extra precautions must be given to older people and people with diabetes to prevent burns in any type of thermal therapy.
Chapter Four – The Influence of Environmental Temperature on the Response of the Skin to Local Pressure: The Impact of Aging and Diabetes
Background: To protect against ischemia, pressure-induced vasodilation (PIV) causes an increase in skin blood flow. Endothelial dysfunction, which is commonly found in older (O) and diabetic (D) patients, and global temperatures can affect the resting blood flow in skin which may reduce the blood flow during and after the application of local pressure. The present study investigated the PIV of the skin with exposure to 3 global temperatures in O and D populations as compared to younger (Y) subjects without diabetes.
Materials and Methods: Older subjects without diabetes (O) (n=15, mean age 64.2±14.0 yrs), subjects with diabetes (D) (n=15, mean age 62.0±5.9 yrs, mean duration 13.2±9.1 yrs), and younger subjects without diabetes (Y) (n=15, mean age 25±2.9 yrs) participated. An infared laser Dopler flow meter was used to measure skin blood flow on the bottom of the foot, lower back, and hand during and after applications of pressure at 7.5, 15, 30, 45, and 60 kPa in a 16°, 24°, and 32°C global temperatures.
Results: The resting blood flow for all subjects was significantly lower in the 16°C environment (p<0.05). D blood flow was significantly lower at rest, during the application of all pressure, and after the release of pressure in all global temperatures (p<0.05). Y showed a significant increase in blood flow after every pressure application, except 7.5 kPa, in all global conditions (p<0.001). O and D did not have a significant reactive hyperemia, especially in the 16°C environment.
Conclusion: The protective mechanism of PIV is severely reduced in older and diabetic populations especially in colder environments where skin blood flow is already diminished.
Background
When pressure is applied to the skin, affected tissues can become hypoxic and build up vasodilator metabolites which dilate the arterioles and decrease the vascular resistance in healthy skin (Wilkin 1987). Once the pressure is released, local blood flow is temporarily elevated due to the reduced vascular resistance. The temporary increase in blood flow is called a reactive hyperemia which serves to reoxygenate the tissue and flush the vasodilator metabolites from the tissue (Minson et al. 2001).
The ability of the skin to protect itself from anoxia during ischemia can be diminished due to endothelial dysfunction (Petrofsky et al. 2008a). This type of vascular dysfunction can diminish the degree of vasodilation associated with a reactive hyperemia. Endothelial dysfunction is an early event and associated with a host of diseases and conditions, such as aging and diabetes. The prognostic value of endothelial dysfunction is just starting to be understood and valued. One way of non-invasively assessing endothelial dysfunction is by looking at endothelium-dependent vasodilation (Endemann and Schiffrin 2004).
One major factor of endothelium-dependent vasodilation is nitric oxide (NO) (Minson et al. 2001, Smits et al. 1995). NO is released by the endothelium in response to exogenous pharmacological substances, platelet-derived factors, and shear stress which promote the release of NO by stimulating nitric oxide synthase (Minson et al. 2001, Dudzinski et al. 2006, Mombouli and Vanhoutte 1999). The NO then causes the smooth muscle surrounding the blood vessel to relax, causing it to vasodilate. Local pressure also causes a temporary increase in blood flow, known as a pressure-induced vasodilation (PIV), which causes a subsequent release of NO (Garry et al. 2005). PIV is a natural protective mechanism allowing the skin blood flow to increase temporarily after local pressure is applied (Fromy et al. 2000, 2002).
Endothelial dysfunction can reduce the body’s ability to produce and/or respond to NO (Endemann and Schiffrin 2004). With damage to the vascular endothelial cells, such as is common in older people (Holowatz et al. 2005), the microcirculation of the skin may not be able to respond to endothelial stressors such as pressure or global temperature (Petrofsky et al. 2008a, Medeiros et al. 2008, Petrofsky 2006). Preliminary data from this laboratory has shown decreased endothelial responses to multiple stressors especially in people with diabetes (Petrofsky et al. 2005a, 2005b, 2006, 2007a, 2007b, 2008b).
Diabetes is an increasingly common disease, with an incidence of at least 10% in the United States, and it has been predicted to reach as high as 30% of the world’s population by the year 2025 (Narayan et al. 2003). Of all the complications associated with diabetes, the most common is damage to the microcirculation and endothelial cells (Rendell and Bamisedun 1992). This vascular endothelial damage results in a reduced ability either to produce NO or a reduced sensitivity to NO (Hogikyan et al. 1998, Caballero et al. 1999). The damaged endothelial cells and adjacent microcirculation may experience diminished PIV in response to local pressure.
Age can also be a factor that may reduce the protective response of PIV. As humans age, the endothelial cells undergo the same type of damage as a person with diabetes; reduced sensitivity to or less NO released in response to external stimuli (Petrofsky et al. 2005a, 2005b, 2005c, 2006). Prior studies indicate there may be a decrease in skin blood flow when low pressures are applied to the skin in diabetic patients, both with and without neuropathies, compared to control subjects (Fromy et al. 2002, 1998, Chan et al. 2000, Peiper 1998). Therefore, a more comprehensive study needs to be done to examine the PIV of subjects with and without diabetes with multiple endothelial stressors.
Changes in global temperature alter resting blood flow in the skin (Petrofsky et al. 2006, Charkoudian 2003). People live in a variety of global temperatures and previous studies on pressure effects on skin blood flow had been on subjects who are younger and people without diabetes in a thermally neutral room because global temperature has been shown to alter resting skin blood flow which may impact endothelial responses to stimuli. Therefore, the purpose of the present study was to investigate the effects of global temperature on endothelial dysfunction by repeating the series in thermally neutral, cool, and warm climates, to examine the effects of resting blood flow on PIV in subjects who were older and who had diabetes. The aim was also to expand upon previous research by examining the reactive hyperemia following local pressure on the foot as compared to the lower back and hand. We hypothesize that subjects who are older and with diabetes will have a reduced PIV response due to endothelial damage, when compared to age-matched non-diabetic and younger subjects. We also hypothesize that the reactive hyperemia associated with PIV will be decreased in a colder environment when compared to the response in a warmer environment.
Materials – Subjects
A total of 45 subjects (3 groups, 15 subjects each) participated (Table 1). The subjects with diabetes must have been diagnosed with type II diabetes for at least three years, been taking the proper medication to control their blood-sugar levels, and have hemoglobin A1c levels between 6-10%. Subjects were excluded if they had any current or past heart problems, high blood pressure (>140 systolic/95 diastolic), or were taking any alpha or beta agonists or antagonists. All protocols and procedures were approved by the Institutional Review Board at Loma Linda University and subjects signed an informed consent document.
There was a
significant difference in the BMI among all 3 groups (p<0.001) (Table 1), with the older diabetic subjects having the highest overall BMIs. There was not a significant difference between the mean ages for the older non-diabetic and diabetic groups (p=0.58). In terms of the Ankle-Brachii Index (ABI), none of the 45 subjects had an ABI score lower than 0.95. The subjects with diabetes had a mean hemoglobin A1c of 7.6 ± 1.1% and had been diagnosed for a mean of 13.2 ± 6.1 years.
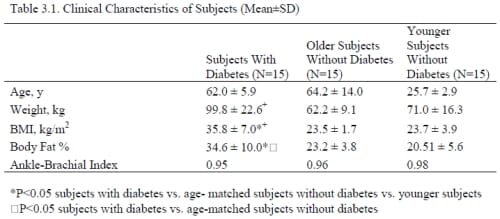
Table 3.1. Clinical Characteristics of Subjects (Mean±SD)
Methods – Control of Environmental Room Temperature
Environmental temperature was controlled using a controlled environment room. The room has thermal homogeneity (laminar flow) with air flow maintained with independent heating and cooling systems capable of controlling room temperature over a range from 0° to 55°C. The application of two independent heating and cooling systems allows rapid changes in room temperature, up to 7°C/minute, and the environmental temperature to be accurately controlled to within +/- 0.5°C. Three room temperatures were used: 16°, 24°, and 32°C. Relative humidity was maintained at 35 ± 10%.
Measurement of Pressure on the Skin
Pressure on the skin was measured through a pressure sensor array (Tactilus Pressure Mapping System, Sensor Products Inc., Madison, NJ) which contained about 500 sensors. The pressure sensors were 7.94×7.94 mm in size and spaced every 42.5×4.5 mm. The thickness of the sensor was 7mm. The sensor used was a resisted strain gauge sensor produced by Sensor Products Inc. (Madison, NJ). The pressure range was 0-68 kPa. The accuracy and reliability was less than 3% error. Calibration was done at the factory as well as randomly throughout the study. The system and software were custom designed for this project to provide a 3 dimensional map of pressure distribution. The array was transduced through a 16 bit A/D converter produced by Sensor Products Inc. with a sample rate of 100K Hz. The three skin sites were the back of the hand between the 2nd and 3rd metacarpal, the lower back 7 cm lateral of the L3-L4 vertebrae, and over the head of the 1st metatarsal on the ball of the foot.
Measurement of Blood Flow
A single point infrared laser Doppler flow meter (TST 140 probe, LDF100 module, Biopac Inc., Goleta, CA) was used to measure the skin blood flow. The skin blood flow was recorded for 30 seconds. The flow meter had a head with an active area of 1 cm2. The probe was plugged into an LDF 100C amplifier and then digitized at 2000 samples per second with a 16 bit analogue to digital converter. The converted signal was then saved for final analysis with a Biopac MP 100 system (Biopac Inc, Goleta, CA).
Measurement of Ankle-Brachial Index (ABI)
Blood pressure measurements were taken at the ankle and brachial artery in the arm using a standard sphygmomanometer cuff and stethoscope while the subject was at rest and had been lying supine for at least 5 minutes. The ankle and arm systolic blood pressure measurements for the left and right limbs were recorded. The ABI is derived by dividing the systolic pressure of the ankle by the systolic pressure of the brachial artery, and the result is used to predict the severity of peripheral arterial disease. The ABI range that is generally considered normal is 0.95 to 1.2.
Measurement of Total Body Fat Percent
The total body fat percent was measured by an impedance plethysmograph (RJL systems, Clinton Twp., MI). The Quantum II provides high-resolution whole body and regional bioelectrical resistance and reactance measurements. The total body fat percent was measured through electrodes placed on the right foot and right hand.
Procedures
On three different days, the subject sat comfortably in the controlled environment room at 16°, 24°, or 32°C for 20 minutes. Before any pressure was applied, baseline blood flow was taken. The laser Doppler flow probe was placed on the ball of foot. The pressure array was placed on top of the probe and manual pressure was applied for 30 seconds while the blood flow was recorded. The pressure was removed for 30 seconds and the blood flow continued to record. Pressure was applied in a random order at 7.5, 15, 30, 45 and 60 kPa. These steps were repeated on the lower back and the back of the hand.
Data Analysis
Calculations of means and standard deviations were done and all values are expressed as mean ± SD. Comparisons among groups were performed using 1-way ANOVA and independent t-tests. The level of significance was p ≤ 0.05. The results were analyzed using SPSS version 15.
Results
Three temperatures were examined; first, a neutral global temperature, then a warm temperature, and finally, a cold global temperature.
Vascular Occlusion of the Foot
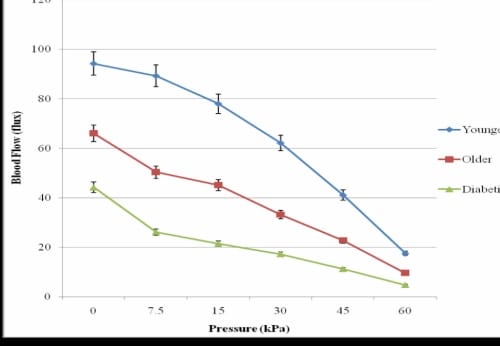
Figure 3.1. This figure illustrates the vascular occlusion on the foot due to application of 30 seconds of pressure in three groups of subjects in 24°C global temperature with standard deviation error bars.
In the thermally neutral global temperature (24°C), when pressure was applied for 30 seconds, the blood flow to the skin of the foot on the younger subjects decreased proportionally to the amount of pressure being applied (Figure 3.1).
The subjects who were older with and without diabetes had a significant decrease in blood flow from the lowest pressure used in this study (7.5 kPa) as compared to baseline blood flows (pO=0.04, pD=0.03). When the greatest pressure used in this study (60 kPa) was applied to the foot, the younger subjects had a blood flow of 17.48±2.63 flux which was significantly higher than the non-diabetic subjects who were older (9.52±1.41 flux, p=0.02) and with diabetes (4.72±0.67 flux, p<0.05). The pattern of response was similar for the hand and lower back.
In terms of the reactive hyperemia following the application of pressure, very light pressure (7.5kPa) on the foot resulted in a significant increase in blood flow for the younger subjects (p=0.03), but not for the subjects who were older with or without diabetes (Figure 3.2).
PIV of the Foot in the Thermally Neutral Global Temperature
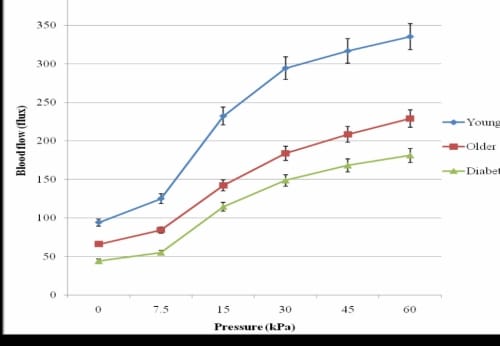
Figure 3.2. PIV of the foot due to application of 30 seconds of pressure for three groups of subjects in 24°C global temperature with standard deviation error bars.
On the lower back and hand, the responses were similar. For the applications of 15, 30, 45, and 60 kPa on the foot, there was not a significant difference between the PIV for the older subjects without diabetes and the subjects with diabetes. When the PIV of the foot and hand were compared, there was no significant difference in the PIV at baseline or for any of the pressure applications. However, the lower back had significantly lower blood flow responses (p=0.02). Figure 3.3 shows a comparison of the blood flow at baseline, 30kPa, and 60kPa for the lower back and foot.
PIV Comparison of the Foot and Lower Back for Three Applications of Pressure
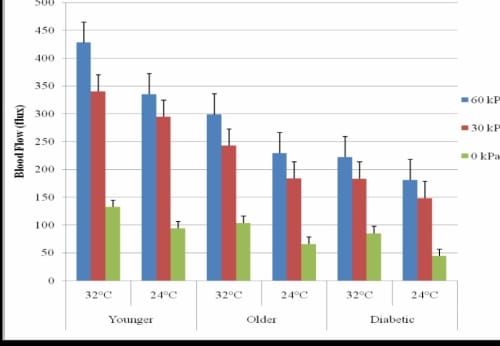
Figure 3.3. This figure illustrates the comparison of PIV of the foot and lower back due to application of three different applications of pressure for three groups of subjects in 24°C global temperature with standard deviation error bars.
32°C Global Temperature
In the warmer environment, 30 seconds of pressure resulted in similarly occluded blood flow as in the 24°C global temperature for the foot, hand, and back. There was a significant increase in resting blood flow for the foot (p=0.02) and hand (p=0.04), but not the back (p=0.17) for all subjects. The lower back did not have a significant increase in resting blood flow (p=0.17) or PIV from any amount of pressure (p=0.36). The blood flow response of heavy pressure (60 kPa) on the foot significantly increased blood flow in the 24°C as compared to the 32°C global temperature (p=0.02) with increases of 27.6% for the younger subjects, 30.3% for the older subjects, and 22.5% for the subjects with diabetes (Figure 3.4). For all other applications of pressure on the foot, there was not a significant increase in blood flow from the 24°C global temperature to the 32°C global temperature for any of the groups of subjects. The response was similar for the hand.
PIV Comparison of the Foot and Lower Back for three Applications of Pressure
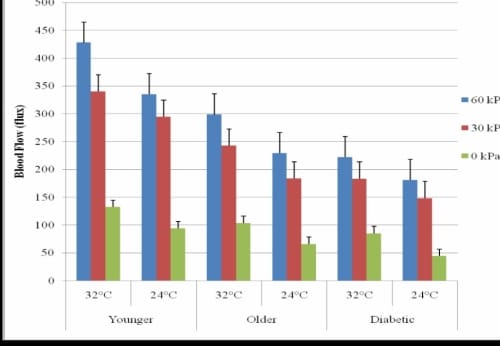
Figure 3.4. This figure illustrates the comparison of PIV of the foot due to application of 30 seconds of pressure for three groups of subjects in 32° and 24°C global temperatures with standard deviation error bars.
16°C Global Temperature
The occlusion resulting from each application of pressure is displayed in Figure 3.5 for the foot for each group of subjects. For the subjects with diabetes, there was not a significant difference between baseline blood flow and the occluded blood flow due to 30 seconds of any of the applications of pressure. The pattern of response was similar for the lower back and hand.
Vascular Occlusion of the Foot in the 16°C Global Temperature
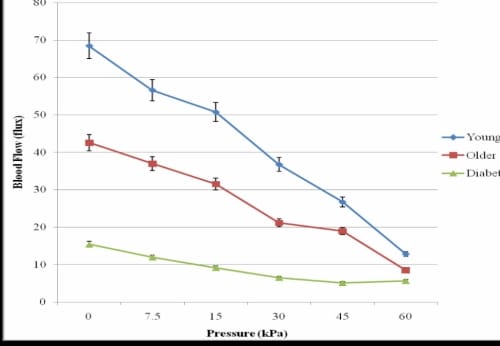
Figure 3.5. This figure illustrates the vascular occlusion on the foot due to application of 30 seconds of pressure in three groups of subjects in 16°C global temperature with standard deviation error bars.
The baseline blood flow of the foot for the subjects with diabetes was 15.42±3.26 flux which was 276% lower than for the older subjects and 443% lower than the blood flow of the younger subjects. This difference in blood flows between the three groups of subjects was statistically significant (p=0.01). The pattern of PIV response to the various amounts of pressure application was similar in the 16°C and 24°C global temperatures. Results were similar for the lower back and hand.
When the PIV for each application of pressure was compared to the baseline blood flow in the 16°C global temperature, the subjects with diabetes had significantly higher increases in blood flow as compared to the age-matched non-diabetic subjects (p=0.01) (Figure 3.6).
In the 24°C global temperature, the subjects with diabetes had a larger percent increase in blood flow from baseline than the older non-diabetic subjects for all of the pressure applications except 7.5 kPa. The 32°C global temperature did not cause the blood flow to increase more in the subjects with diabetes than the older non-diabetic subjects for any application of pressure, however, the differences were not significant for the blood flow and percent blood flow increases for the subjects who were older without diabetes as compared to the subjects with diabetes.
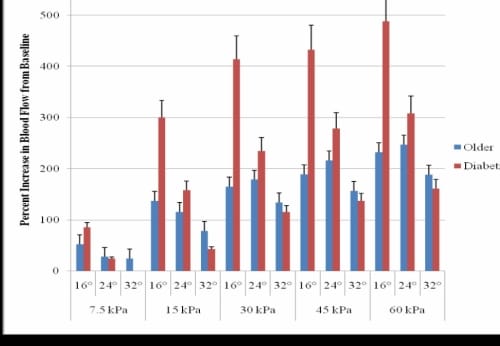
Figure 3.6. This figure illustrates the percent increase in blood flow from baseline of older age-matched diabetic and non-diabetic subjects for all pressure amounts in three global temperatures with standard deviation error bars.
Discussion
When pressure is applied to the skin, blood flow is temporarily reduced or occluded. Once pressure is released, the pressure-induced vasodilation (PIV) occurs to reoxygenate the skin and tissues through briefly vasodilated skin blood flow (Wilkin 1987). Endothelial dysfunction, such as is common in people who are older or who have diabetes (Endemann and Schiffrin 2004, Petrofsky et al. 2006, 2007b, Charkoudian 2003), may reduce the PIV blood flow (Petrofsky et al. 2008a, Fromy et al. 2000, 2002) and affected tissues may not be reoxygenated properly. The vascular endothelial cells may be stressed by changes in global temperatures which may further reduce the reactive hyperemia associated with PIV (Petrofsky et al. 2006, 2008a).
In the present investigation, for the younger subjects, the pattern of response of pressure versus blood flow was similar after exposure to all three global temperatures. Overall, the pattern of response of the decreased blood flow was similar for the foot, lower back, and hand. For the PIV, in the 24° and 32°C environments, the lines were nearly parallel for the subjects who were older and who had diabetes. The diabetic subjects had a blood flow response that was a mean of 29 flux lower in the 24°C and 51 flux lower than the older subjects in the 32°C global temperatures. In the 16°C environment, the pattern of response was similar for the older and younger non-diabetic subjects, however, the subjects with diabetes had a blood flow response that was significantly lower than the younger and older subjects for all applications of pressure, except 60 kPa. The foot and hand had similar PIV responses while the lower back had a similar pattern but an overall reduced blood flow by a mean of 50 flux. Although the baseline blood flow for the subjects with diabetes was significantly lower in the 16° and 24°C global temperatures, they had a larger percent increase in blood flow as a result of the 15, 30, 45, and 60 kPA applications of pressure than the older non-diabetic subjects. This suggests that local pressure may help to cause temporary increases in blood flow which may be useful in therapy and treatment plans for diabetic populations.
Previous studies from this laboratory have shown the response of vascular endothelial cells to stimuli, such as global temperature or pressure, is affected by stimuli from their environment (Petrofsky (Petrofsky et al. 2005a, 2005b, 2008a, 2008c, 2008d).
Similar to data presented here, skin blood flow was decreased at low pressure applications in subjects with diabetes (Fromy 2000) which indicates an inability of the skin to respond to small amounts of stress to the microcirculation and endothelial cells. Chronic pressure applications have been shown to further reduce the PIV mechanism in patients with diabetes, suggesting C-fibers are involved in cutaneous vasodilation (Fromy 2000). Three main biochemical pathways may decrease NO availability and increase endothelial dysfunction: the polyol pathway, nonenzyme glycation, and redox potential alterations (Chan et al. 2000, Brownlee 2001). NO production can be impaired by increased flux of the polyol pathway which can completely decrease NADPH (Greene et al. 1978, Soriano et al. 2001, Stevens et al. 1994, Cameron et al. 1996, 1997). Advanced glycation end product accumulation reduces NO bioavailability (Chan et al. 2000, Brownlee 2001) and has been seen in early stages of diabetes (Siggaudo-Roussel et al. 2004) which suggests endothelial alterations had already started.
Global and skin temperatures may also play an important role in determining the magnitude of PIV. The present data shows the colder environment resulted in significantly reduced blood flow due to pressure applications and PIV once the pressure was removed for all groups of subjects. This suggests PIV mechanisms may be receptive to mechanothermal nervous receptors as well as biomechanical (Fromy et al. 2002). A recent study from this laboratory showed vasodilation was attenuated in skin that had been both locally and globally heated (McLellan et al. 2008).
Conclusions
The PIV mechanism was reduced in the colder 16°C global temperature in the older subjects without diabetes, and most significantly, in the subjects with diabetes regardless of the environmental temperature. The PIV response seems to be highly sensitive to endothelial NO levels. Older people and people with diabetes may have damaged endothelial cells causing this response to be absent or diminished. PIV could be a non-invasive indicator of endothelial dysfunction that can be used to diagnose patients that may be predisposed to microvascular problems such as pressure ulcers.
Limitations
One of the limitations of this study is that we did not apply pressure less than 7.5 kPa. It is possible that people with diabetes will have a reactive hyperemia to lighter forces of pressure such as 3 kPa, but that was not examined in this study.
Chapter 5 – Conclusion
When the skin is exposed to heat, the resulting skin temperatures cannot be accurately predicted in people who are older and who have diabetes because the Pennes Model does not account for endothelial dysfunction, subcutaneous fat, and skin moisture. In people who are older and with diabetes, endothelial dysfunction may be present as well as thicker subcutaneous fat and drier skin, all of which will account for an impaired ability to dissipate heat. For example, when a local heat stress is applied to the skin, the change in skin temperature over 5 seconds for subjects with diabetes was 176% higher than the older non-diabetic subjects and 329% higher than the younger non-diabetic subjects in the 16°C global temperature. As another example, resting blood flow increased due to increased sympathetic activity in the 32°C global temperature. The subjects with diabetes had 209% and 354% higher increases in skin temperature than the older and younger non-diabetic subjects, respectively.
When two stressors are applied to the endothelial cells simultaneously, such as when the skin is exposed to both local and global heat, the two stressors caused a potentiated response in the younger non-diabetic subjects. For the older subjects, with and without diabetes, not only was the blood flow response of the older subjects with and without diabetes lower, but the blood flow seemed to plateau due to the combination of multiple stressors. The notion that there would simply be a reduced response is now shown to be incorrect. The data shows a plateau similar to data collected from a previous study in this laboratory when the endothelial cells were stressed with both global heat and orthostatic stress (Petrofsky et al. 2008). The same pattern of response was found when the interaction of local pressure and global heat was examined. For the older subjects and subjects with diabetes, there was a significant decrease in blood flow from even the smallest amount of pressure (7.5 kPa). In terms of the pressure-induced vasodilation, very light pressure on the foot resulted in a significant increase in blood flow for the younger subjects, but not for the subjects who were older, with or without diabetes. This indicates an inability of the endothelial cells to react to the combination of shear stress through local pressure and global heat.
Thus, multiple endothelial stressors inhibit the response of the endothelium causing absent or reduced vasodilation in response to local heat, global heat, and/or pressure applications in people with endothelial dysfunction. In addition, we have seen from these studies, that for older people, with and without diabetes, the current Pennes model does not accuarately predict limb temperatures because it does not account for the factors of endothelial dysfunction, thicker subcutaneous fat, and drier skin. These results indicate a need for further research to expand the Pennes model and equation.
References
- Ardevol A, Adan C, Remesar X, Fernandez-Lopez JA, Alemany M. Hind leg heat balance in obese Zucker rates during exercise. Pflugers Arch. 1998;435(4):454- 64.
- Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. Am J Physiol Lung Cell Mol Physiol. 2007 Jun;292(6):L1515-25.
- Bellien J, Thuillez C, Joannides R. Role of endothelium-derived hyperpolarizing factor in the regulation of radial artery Basal diameter and endothelium-dependent dilatation in vivo. Clin Exp Pharmacol Physiol. 2008 Apr;35(4):494-7.
- Benabdallah H, Messaoudi D, Gharzouli K. The spontaneous mechanical activity of the circular smooth muscle of the rabbit colon in vitro. Pharmacol Res.2008 Jan 16 Bessey PQ, Aron RR, DiMaggio CJ, Yurt RW. The vulnerabilities of age: burns in children and older adults. Surgery. 2006; 140: 705-17.
- Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996; 17(2): 95-107. Brink H, Werner J. Efficiency function: improvement of classical bioheat approach. J Appl Physiol. 1994;77:4, 1617-1622.
- Carmeli E, Hagar P, Coleman R. The aging hand. J Gerontol A Biol Sci Med. 2003;58:146-152.
- Charkoudian K. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clinic Protocol. 2003;78:603-12.
- Chato, J. Heat transfer to blood vessels. J Biomech. Eng. 1980;102: 110-118. Chen, M. M., and K. R. Holmes. Microvascular contributions in tissue heat transfer. Ann NY Acad. Sci. 1980; 335: 137-150.
- Comerford A, Plank MJ, David T. Endothelial nitric oxide synthase and calcium production in arterial geometries: an integrated fluid mechanics/cell model. J Biomech Eng. 2008 Feb;130(1):011010.
- Edholm OG, Fox RH, Macpherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol. 1956;134:612-619.
- Edholm OG, Fox RH, Macpherson RK. Vasomotor control of the cutaneous blood vessels in the human forearm. J Physiol. 1957; 139:455-465. Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64:617-628.
- Guler A, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoke activation of the ion channel, TRVP4. J Neurosci. 2002;22:6408-6414.
- Ho CW, Beard JL, Farrell PA, Minson CT, and Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol. 1990;82(4): 1126-1135. Holowatz LA, Thompson CS, Minson CT, and Kenney WL. Mechanisms of acetylcholine-mediated vasodilation in young and aged human skin. J Physiol. 2005;563:965-973.
- Houghton BL, Meendering JR, Wong BJ, and Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex reponse to gradual local heating in human skin. J Physiol. 2006;572:811-820. Huang PL. Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep. 2003 Dec;5(6):473-80.
- Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6:337-346.
- Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824-829.
- Kellogg DL, Morris SR, Rodriquez SB, Liu Y, Grossmann M, Stagni G, and Shepherd AM. Thermoregulatory reflexes and cutaneous active vasodilation during heat stress in hypertensive humans. J Appl Physiol. 1998;85(1): 175-180.
- Kellogg DL. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Phyiol. 2006;100:1709-18. Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta. 2007;1772(8):907-14.
- Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007 Mar 30;282(13):9364-71.
- Maglinger PE, Sessler DI, Lenhardt R. Cutaneous heat loss with three surgical drapes, one impervious to moisture. Aneth Analg. 2005;100(3):738-42.
- Medeiros JV, Gadelha GG, Lima SJ, Garcia JA, Soares PM, Santos AA, Brito GA, Ribeiro RA, Souza MH. Role of the NO/cGMP/K(ATP) pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br J Pharmacol. 2008 Feb;153(4):721-7.
- Mehert P, Malchaire J, Kampmann B, Piette A, Griefahn B, Gebhardt H. Prediction of the average skin temperature in warm and hot environments. Eur J Appl Physiol. 2002;82:52-60.
- Minson CT, Berry LT, and Joyner MJ. Nitric oxide and neutrally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619-1629. Nelson DA. Invited editorial on “Pennes’ 1948 paper revisited”. J Appl Physiol 1998;85: 2-3.
- Ou J, Ou Z, Ackerman AW, Oldham KT, Pritchard KA Jr. Inhibition of heat shock protein 90 (hsp90) in proliferating endothelial cells uncouples endothelial nitric oxide synthase activity. Free Radic Biol Med. 2003 Jan 15;34(2):269-76. Pandolf KB. Aging and heat tolerance at rest or during work. Exp Aging Res. 1991; 17(3): 189-203.
- Pennes H. Analysis of tissue and arterial blood temperature in the resting human forearm. J Appl Physiol. 1948;1:93–122
- Pergola PE, Kellogg DL, Johnson JM, Kosiba WA, and Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol 1993;265: H785-H792.
- Petrofsky JS, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the forearm; impact of rosiglitazone. BMC Endocrine Disorders. 2005;5:4-9.
- Petrofsky JS, Prowse M, Lohman E. The influence of aging and diabetes on skin and subcutaneous fat thickness in different regions of the body. J Appl Res. 2008 [in press].
- Petrofsky JS and Laymon D. Heat transfer to deep tissue; effect of body fat and heating modality. Sub J Med eng Tech. 2008 [in press]. Petrofsky JS and Lind AR. Insulative power of body fat on deep muscle temperatures and isometric endurance. J Appl Physiol. 1975;39:639-642. Petrofsky JS and Lind AR. The relationship of body fat content to deep muscle temperature and isometric endurance in man. Clin Sci Mol Med. 1975;48:405- 412.
- Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and the response of the vascular endothelial cells. Med Sci Monit. 2008 [in press]. Petrofsky JS, De la Cuesta Z, Labial L et al: The effects of contrast baths on skin blood flow; the influence of room temperature and diabetes. Med Sci Monit. 2006;12(7):CR290-95
- Petrofsky JS, Lee S, Cuneo-Librona M, Apodaca P. The effect of rosiglitazone on orthostatic tolerance during heat exposeure in individuals with type II diabetes. Diabetes Technol Ther. 2007 Aug;9(4):377-86.2005a
- Petrofsky JS, Lee S, Cuneo-Librona M. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monit. 2005;11:CR562-69.
- Petrofsky JS, Lee S, Patterson C, Cole M, Stewart B. Sweat production during global heating and during isometric exercise in people with diabetes. Med Sci Monit. 2005;11:CR515-7.
- Petrofsky JS, Lohman E 3rd, Lee S, de la Cuesta Z, Labial L, Iouciulescu R, Moseley B, Malty AA. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory Pract. 2007 Jul-Aug;23(4):189-97.
- Petrofsky JS, McLellan K, Bains G, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E, Schwab E. The influence of ageing on the ability of the skin to dissipate heat. Med Sci Mon. 2008 [in press].
- Petrofsky JS, McLellan K, Bains G, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E. The influence of diabetes on the ability of the skin to dissipate heat; the impact of skin thickness and subcutaneous fat. J Appl Res. 2008b [in press]. Petrofsky JS, McLellan K, Bains G, Prowse M. The influence of age, diabetes, and obesity on skin blood flow response to applied pressure. Diabetes Technol Ther, 2008 [in press].
- Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol. 1977;43:133-137.
- Rowell LB. Cardiovascular adjustments to thermal stress. In: Handbook of Physiology. Shepherd JT, Abboud FM, eds. Section 2: The Cardiovascular System. Vol 3, pt 2. Bethesda, Md: American Physiological Society; 1983:967-1023.
Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986. - Shvartz E, Bhattacharya A, Sperinde SJ, Brock PJ, Sciaraffa D, Van Beaumont W. Sweating responses during heat acclimation and moderate conditioning. J Appl Physiol.1979;46(4):675-80.
- Siddappa K. Dry skin conditions, eczema, and emollients in their management. Indian J Dermatol Venereol Leprol 2003;69:69-75
- Stransberry KB, Peppard HR, Babyak LM, Popp G, McNitt PM, Vinik AI. Primary nociceptive afferents mediate the blood flow dysfunction in non-glaborour (hairy) skin of type 2 diabetes: a new model for the pathogensesis of microvascular dysfunction. Diabetes Care 1999;22:1549-1554.
- Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007 Dec;293(6):L1444-53
- Swiecki C, Stojadinovic A, Anderson J, Zhao A, Dawson H, Shea-Donohue T. Effect of hyperglycemia and nitric oxide synthase inhibition on heat tolerance and induction of heat shock protein 72 kDa in vivo. Am Surg. 2003 Jul;69(7):587-92
- Watanabe H, Vriens J, Suh HS,Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 2002;277(49):47044-47051.
- Webb P. Temperatures of skin, sub-cutaneous tissue, muscle and core in resting men in cold, comfortable, and hot conditions. Eur J Appl Physiol Occup Physiol. 1992;64:471-476.
- Wissler, EH. Pennes’ 1948 paper revisited. J Appl Physiol. 1985: 36-42, 1998. Bellien J, Thuillez C, Joannides R. Role of endothelium-derived hyperpolarizing factor in the regulation of radial artery Basal diameter and endothelium-dependent dilatation in vivo. Clin Exp Pharmacol Physiol. 2008 Apr;35(4):494-7.
- Benabdallah H, Messaoudi D, Gharzouli K. The spontaneous mechanical activity of the circular smooth muscle of the rabbit colon in vitro. Pharmacol Res. 2008 Jan 16 [Epub ahead of print]
- Branchet MC, Boisnic S, Frances C, Robert AM. Shin thickness changes in normal aging skin. Gerontology. 1990;36(1):28-35.
- Brink H, Werner J. Efficiency function: improvement of classical bioheat approach. J Appl Physiol. 1994;77:4, 1617-1622.
- Brock SC, Tonussi CR. Intrathecally injected morphine inhibits inflammatory paw edema: the involvement of nitric oxide and cyclic-guanosine monophosphate. Anesth Analg. 2008 Mar;106(3):965-71.
- Charkoudian K. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clinic Protocol. 2003;78:603-12.
- Comerford A, Plank MJ, David T. Endothelial nitric oxide synthase and calcium production in arterial geometries: an integrated fluid mechanics/cell model. J Biomech Eng. 2008 Feb;130(1):011010.
- Ho CW, Beard JL, Farrell PA, Minson CT, and Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl. Physiol. 1997Petrofs;82(4): 1126- 1135.
- Houghton BL, Meendering JR, Wong BJ, and Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811-820.
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. Handbook of Physiology. Bethesda, MD: Am. Physiol. Soc. 1996.
- Kellogg DL Jr, Pergola PA, Peist KL, Kosiba WA, Crandall CG, Grossmann M, and Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 1995;77:1222-1228.
- Kellogg DL, Morris SR, Rodriquez SB, Liu Y, Grossmann M, Stagni G, and Shepherd AM. Thermoregulatory reflexes and cutaneous active vasodilation during heat stress in hypertensive humans. J Appl. Physiol. 1998;85(1): 175-180.
- Kellogg DL. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Phyiol. 2006;100:1709-18. Kenney WL, Hodgson JL. Heat tolerance, thermoregulation and ageing. Sports Med. 1987 Nov-Dec;4(6):446-56.
- Kenney WL. Human cardiovascular responses to passive heat stress. J Physiol. 2008;586(1):3
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising med during acute sympathetic stimulation. J Physiol 2003;55:337-344. Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochem Biophys Acta. 2007 Aug;1992(8):907-14.
- McCord G, Cracowski JL, Minson C. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Intregr Comp Physiol. 2006; 291: R596-R602.
- Medeiros JV, Gadelha GG, Lima SJ, Garcia JA, Soares PM, Santos AA, Brito GA, Ribeiro RA, Souza MH. Role of the NO/cGMP/K(ATP) pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br J Pharmacol. 2008 Feb;153(4):721-7.
- Minson CT, Berry LT, and Joyner MJ. Nitric oxide and neutrally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619-1629. Pennes H. Analysis of tissue and arterial blood temperature in the resting. 1948.
- Pergola PE, Kellogg DL, Johnson JM, Kosiba WA, and Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol. 1993;265:H785-H792.
- Petrofsky J, Al-Malty AM, Prowse M. Relationship between multiple stimuli and the response of the vascular endothelial cells. Med Sci Monit. 2008c [in press].
- Petrofsky J, McLellan K, Bains G, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E. The influence of diabetes on the ability of the skin to dissipate heat; the impact of skin thickness and subcutaneous fat. J Appl Res. 2008b [in press].
- Petrofsky J, McLellan K, Bains G, Prowse M. The influence of age, diabetes, and obesity on skin blood flow response to applied pressure. Diabetes Technol Ther. 2008a [in press].
- Petrofsky JS, De la Cuesta Z, Labial L et al: The effects of contrast baths on skin blood flow; the influence of room temperature and diabetes. Med Sci Monit, 2006; 12(7): CR290-95
- Petrofsky JS, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the forearm; impact of rosiglitazone. BMC Endocrine Disorders. 2005a;5:4-9.
- Petrofsky JS, Lee S, Cuneo-Librona M, Apodaca P. The effect of rosiglitazone on orthostatic tolerance during heat exposure in individuals with type II diabetes. Diabetes Technol Ther. 2007a Aug;9(4):377-86.
- Petrofsky JS, Lee S, Cuneo-Librona M. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monit. 2005b;11:CR562-69.
- Petrofsky JS, Lohman E 3rd, Lee S, de la Cuesta Z, Labial L, Iouciulescu R, Moseley B, Malty AA. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory
- Pract. 2007b Jul-Aug;23(4):189-97.
- Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986.
- Sagawa S, Shiraki K, Yousef MK, Miki K. Sweating and cardiovascular responses of aged men to heat exposure. J Gerontol. 1988 Jan;43(1):M1-8.
- Schalkwijk CG, Stehouwer C. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005:109;143-159.
- Schiffrin EL. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J Cardiovasc Pharmacol 2001;38[Suppl 2]:S3-S6.
- Stransberry KB, Peppard HR, Babyak LM, Popp G, McNitt PM, Vinik AI. Primary nociceptive afferents mediate the blood flow dysfunction in non-glaborous (hairy) skin of type 2 diabetes: a new model for the pathogenesis of microvascular dysfunction. Diabetes Care 1999;22:1549-1554.
- Tarif N, Bakris G. The place for calcium channel blockers in the treatment of hypertension in patients with diabetes mellitus. Saudi J Kidney Dis Transpl. 2000 Oct-Dec;11(4):531-6.
- Verma S and Anderson TL. Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002;105:546-549.
- Watanabe H, Vriens J, Suh HS, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 2002;277(49):47044-47051.
- Weigert AL, Martin PY, Niederberger M, Higa EM, McMurtry IF, Gines P, Schrier RW. Endothelium-dependent vascular hyporesponsiveness without detection of nitric oxide synthase induction in aortas of cirrhotic rats. Hepatology. 1995 Dec:22(6):1856-65.
- Wissler EH. Pennes’ 1948 paper revisited. J Appl Physiol. 1998;85(1):35-41. Medeiros JV, Gadelha GG, Lima SJ, Garcia JA, Soares PM, Santos AA, Brito GA, Ribeiro RA, Souza MH. Role of the NO/cGMP/K(ATP) pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br J Pharmacol. 2008 Feb;153(4):721-7.
- Petrofsky JS, De la Cuesta Z, Labial L et al: The effects of contrast baths on skin blood flow; the influence of room temperature and diabetes. Med Sci Monit. 2006;12(7):CR290-95
- Petrofsky JS, Lee S, Cuneo-Librona M. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monit. 2005a;11:CR562-69.
- Petrofsky JS, Lee S, Patterson C, Cole M, Stewart B. Sweat production during global heating and during isometric exercise in people with diabetes. Med Sci Monit. 2005b;11:CR515-7.
- Petrofsky JS, Lee S, Cuneo-Librona M, Apodaca P. The effect of rosiglitazone on orthostatic tolerance during heat exposeure in individuals with type II diabetes. Diabetes Technol Ther. 2007a Aug;9(4):377-86.
- Petrofsky JS, Lohman E 3rd, Lee S, de la Cuesta Z, Labial L, Iouciulescu R, Moseley B, Malty AA. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory Pract. 2007b Jul-Aug;23(4):189-97.
- Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and the response of the vascular endothelial cells. Med Sci Monit. 2008b [in press]. Narayan V, Boyle JP, Thompson TJ, Sorenson SW, Williamson DF. JAMA. 2003;290:1884-1890.
- Rendell M, Bamisedun O. Skin blood flow and current perception in pentoxifyllinetreated diabetic neuropathy. Angiology. 1992;43(10):843-51.
- Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol and Metabol. 1998;83:1946-51.
- Caballero AE, Arora S, Saouaf R. Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856-1862.
- Petrofsky JS, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the
forearm; impact of rosiglitazone. BMC Endocrine Disorders. 2005c;5:4. - Fromy B, Abraham P, Aumet JL. Non-nociceptive capsaisin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 811: 166-168, 1998.
- Chan NN, Valance P, Colhoum HM. Nitric oxide and vascular responses in type I diabetes. Diabetologia. 2000;43:137-147.
- Peiper GM. Review of alteration in endothelial nitric oxide production in diabetes. Protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047-1060.
- Charkoudian K. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clinic Protocol. 2003;78:603-12. Petrofsky JS, McLellan K, Bains G, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E, Schwab E. The influence of ageing on the ability of the skin to dissipate heat. Med Sci Mon. 2008c [in press].
- Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and the response of the vascular endothelial cells. Med Sci Monit. 2008d [in press]. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
- Greene DA, Chakrabarti S, Lattimer SA, Sima AA. Role of sorbitol accumulation and myo-inositol depletion in paramodal swelling of large myelinated nerve fibers in the insulin-deficient spontaneously diabetic bio-breeding rat: reversal of insulin replacement, an aldose reductase inhibitor, and myo-inositol. J Clin Invest. 1978:;79;1479-1485.
- Soriano FG, Virag L, Jactap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KGK, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly (ADP-ribose) polymerase activation. Nat Med. 2001;7:108-113.
- Stevens MJ, Dananberg J, Feldman EL, Lattimer SA, Kamijo M, Thomas TP, Shindo H, Sima AA, Greene DA. The linked roles of nitric oxide, aldose reductase, and (Na+, K+)-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J Clin Invest. 1994;94:853-859.
- Cameron NE, Cotter MA, Dines KC, Hohman TC. Reversal of defective peripheral nerve conduction velocity, nutritive endoneurial blood flow, and oxygenation by a novel aldose reductase inhibitor, WAY-121-509, in streptozotocin-induced diabetic rats. J Diabetes Complications. 1996;10:43-53.
- Sigaudo-Roussel D, Demoit C, Fromy B, Koitka A, Leftheroitis G, Abraham P, Saumet JL. Early endothelial dysfunction severely impairs skin blood flow repsonse to local pressure application in streptozotocin-induced diabetic mice. Diabetes. 2004;53:1564-1569.
- McLellan K, Petrofsky JS, Zimmerman G, Prowse M, Bains G, Lee S. Multiple stressors and the response of the vascular endothelial cells; the effect of aging and diabetes. Med Eng Phys. [submitted].
- Ardevol A, Adan C, Remesar X, Fernandez-Lopez JA, Alemany M. Hind leg heat balance in obese Zucker rates during exercise. Pflugers Arch. 1998;435(4):454- 64.
- Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. Am J Physiol Lung Cell Mol Physiol. 2007 Jun;292(6):L1515-25.
- Bellien J, Thuillez C, Joannides R. Role of endothelium-derived hyperpolarizing factor in the regulation of radial artery Basal diameter and endothelium-dependent dilatation in vivo. Clin Exp Pharmacol Physiol. 2008 Apr;35(4):494-7.
- Benabdallah H, Messaoudi D, Gharzouli K. The spontaneous mechanical activity of the circular smooth muscle of the rabbit colon in vitro. Pharmacol Res. 2008 Jan 16 [Epub ahead of print]
- Bessey PQ, Aron RR, DiMaggio CJ, Yurt RW. The vulnerabilities of age: burns in children and older adults. Surgery. 2006; 140: 705-17.
- Branchet MC, Boisnic S, Frances C, Robert AM. Shin thickness changes in normal aging skin. Gerontology. 1990;36(1):28-35.
- Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996; 17(2): 95-107. Brink H, Werner J. Efficiency function: improvement of classical bioheat approach. J Appl Physiol. 1994;77:4, 1617-1622.
- Brock SC, Tonussi CR. Intrathecally injected morphine inhibits inflammatory paw edema: the involvement of nitric oxide and cyclic-guanosine monophosphate. Anesth Analg. 2008 Mar;106(3):965-71.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
- Caballero AE, Arora S, Saouaf R. Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856-1862.
- Cameron NE, Cotter MA, Dines KC, Hohman TC. Reversal of defective peripheral nerve conduction velocity, nutritive endoneurial blood flow, and oxygenation by a novel aldose reductase inhibitor, WAY-121-509, in streptozotocin-induced diabetic rats. J Diabetes Complications. 1996;10:43-53.
- Carmeli E, Hagar P, Coleman R. The aging hand. J Gerontol A Biol Sci Med. 2003;58:146-152.
- Chan NN, Valance P, Colhoum HM. Nitric oxide and vascular responses in type I diabetes. Diabetologia. 2000;43:137-147.
- Charkoudian K. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clinic Protocol. 2003;78:603-12.
- Chato, J. Heat transfer to blood vessels. J Biomech. Eng. 1980;102: 110-118. Chen, M. M., and K. R. Holmes. Microvascular contributions in tissue heat transfer. Ann NY Acad. Sci. 1980; 335: 137-150.
- Comerford A, Plank MJ, David T. Endothelial nitric oxide synthase and calcium production in arterial geometries: an integrated fluid mechanics/cell model. J Biomech Eng. 2008 Feb;130(1):011010.
- Edholm OG, Fox RH, Macpherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol. 1956;134:612-619.
- Edholm OG, Fox RH, Macpherson RK. Vasomotor control of the cutaneous blood vessels in the human forearm. J Physiol. 1957; 139:455-465.
- Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64:617-628.
- Fromy B, Abraham P, Aumet JL. Non-nociceptive capsaisin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 811: 166-168, 1998.
- Greene DA, Chakrabarti S, Lattimer SA, Sima AA. Role of sorbitol accumulation and myo-inositol depletion in paramodal swelling of large myelinated nerve fibers in the insulin-deficient spontaneously diabetic bio-breeding rat: reversal of insulin replacement, an aldose reductase inhibitor, and myo-inositol. J Clin Invest. 1978:;79;1479-1485.
- Guler A, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoke activation of the ion channel, TRVP4. J Neurosci. 2002;22:6408-6414. Ho CW, Beard JL, Farrell PA, Minson CT, and Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl. Physiol. 1997Petrofs;82(4): 1126- 1135.
- Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol and Metabol. 1998;83:1946- 51.
- Holowatz LA, Thompson CS, Minson CT, and Kenney WL. Mechanisms of acetylcholine-mediated vasodilation in young and aged human skin. J Physiol. 2005;563:965-973.
- Houghton BL, Meendering JR, Wong BJ, and Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex reponse to gradual local heating in human skin. J Physiol. 2006;572:811-820. Huang PL. Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep. 2003 Dec;5(6):473-80.
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. Handbook of Physiology. Bethesda, MD: Am. Physiol. Soc.1996.
- Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6:337-346.
- Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824-829.
- Kellogg DL Jr, Pergola PA, Peist KL, Kosiba WA, Crandall CG, Grossmann M, and Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 1995;77:1222-1228.
- Kellogg DL, Morris SR, Rodriquez SB, Liu Y, Grossmann M, Stagni G, and Shepherd AM. Thermoregulatory reflexes and cutaneous active vasodilation during heat stress in hypertensive humans. J Appl Physiol. 1998;85(1): 175-180.
- Kellogg DL. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Phyiol. 2006;100:1709-18. Kenney WL, Hodgson JL. Heat tolerance, thermoregulation and ageing. Sports Med. 1987 Nov-Dec;4(6):446-56.
- Kenney WL. Human cardiovascular responses to passive heat stress. J Physiol. 2008;586(1):3
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising med during acute sympathetic stimulation. J Physiol 2003;55:337-344.
- Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochem Biophys Acta. 2007 Aug;1992(8):907-14.
- Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007 Mar 30;282(13):9364-71. Maglinger PE, Sessler DI, Lenhardt R. Cutaneous heat loss with three surgical drapes, one impervious to moisture. Aneth Analg. 2005;100(3):738-42.
- McCord G, Cracowski JL, Minson C. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Intregr Comp Physiol. 2006; 291: R596-R602.
- McLellan K, Petrofsky JS, Zimmerman G, Prowse M, Bains G, Lee S. Multiple stressors and the response of the vascular endothelial cells; the effect of aging and diabetes. Med Eng Phys. [submitted].
- Mehnert P, Malchaire J, Kampmann B, Piette A, Griefahn B, Gebhardt H. Prediction of the average skin temperature in warm and hot environments. Eur J Appl Physiol. 2002;82:52-60.
- Narayan V, Boyle JP, Thompson TJ, Sorenson SW, Williamson DF. JAMA. 2003;290:1884-1890.
- Ou J, Ou Z, Ackerman AW, Oldham KT, Pritchard KA Jr. Inhibition of heat shock protein 90 (hsp90) in proliferating endothelial cells uncouples endothelial nitric oxide synthase activity. Free Radic Biol Med. 2003 Jan 15;34(2):269-76. Pandolf KB. Aging and heat tolerance at rest or during work. Exp Aging Res. 1991; 17(3): 189-203.
- Peiper GM. Review of alteration in endothelial nitric oxide production in diabetes. Protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047-1060.
- Pergola PE, Kellogg DL, Johnson JM, Kosiba WA, and Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol 1993;265: H785-H792.
- Petrofsky J, McLellan K, Bains G, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E. The influence of diabetes on the ability of the skin to dissipate heat; the impact of skin thickness and subcutaneous fat. J Appl Res. 2008 [in press].
- Petrofsky JS and Lind AR. Insulative power of body fat on deep muscle temperatures and isometric endurance. J Appl Physiol. 1975;39:639-642.
- Petrofsky JS and Lind AR. The relationship of body fat content to deep muscle temperature and isometric endurance in man. Clin Sci Mol Med. 1975;48:405- 412.
- Petrofsky JS, Al-Malty AM, Prowse M. Relationship between multiple stimuli and the response of the vascular endothelial cells. Med Sci Monit. 2008 [in press].
- Petrofsky JS, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the forearm; impact of rosiglitazone. BMC Endocrine Disorders. 2005a;5:4-9.
- Petrofsky JS, Lee S, Cuneo-Librona M, Apodaca P. The effect of rosiglitazone on orthostatic tolerance during heat exposeure in individuals with type II diabetes. Diabetes Technol Ther. 2007 Aug;9(4):377-86.2005a
- Petrofsky JS, Lee S, Cuneo-Librona M. The impact of rosiglitazone on heat tolerance in patients with type 2 diabetes. Med Sci Monit. 2005;11:CR562-69.
- Petrofsky JS, Lohman E 3rd, Lee S, de la Cuesta Z, Labial L, Iouciulescu R, Moseley B, Malty AA. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory Pract. 2007 Jul-Aug;23(4):189-97.
- Petrofsky JS, Lohman E 3rd, Suh HJ, Garcia J, Ander A, Sutterfield C, Grabicki J, Khandge C. Determintation of the conductive heat exchange of the skin in relation to environmental temperature. J Appl Res. 2006;6(2):157-169.
- Petrofsky JS, Lohman E 3rd, Suh HJ, Garcia J, Ander A, Sutterfield C, Khandge C. The effects of aging on conductive heat exchange in the skin at two environmental temperatures. Med Sci Mon. 2006;12(10):CR400-408.
- Petrofsky JS, McLellan K, Bains G, Prowse M. The influence of age, diabetes, and obesity on skin blood flow response to applied pressure. Diabetes Technol Ther, 2008 [in press].
- Petrofsky JS, Prowse M, Lohman E. The influence of aging and diabetes on skin and subcutaneous fat thickness in different regions of the body. J Appl Res. 2008 [in press].
- Rowell LB. Cardiovascular adjustments to thermal stress. In: Handbook of Physiology. Shepherd JT, Abboud FM, eds. Section 2: The Cardiovascular System. Vol 3, pt 2. Bethesda, Md: American Physiological Society; 1983:967-1023.
- Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986.
- Sagawa S, Shiraki K, Yousef MK, Miki K. Sweating and cardiovascular responses of aged men to heat exposure. J Gerontol. 1988 Jan;43(1):M1-8.
- Schalkwijk CG, Stehouwer C. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005:109;143-159.
- Schiffrin EL. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J Cardiovasc Pharmacol 2001;38[Suppl 2]:S3-S6. Shvartz E, Bhattacharya A, Sperinde SJ, Brock PJ, Sciaraffa D, Van Beaumont W. Sweating responses during heat acclimation and moderate conditioning. J Appl Physiol.1979;46(4):675-80.
- Siddappa K. Dry skin conditions, eczema, and emollients in their management. Indian J Dermatol Venereol Leprol 2003;69:69-75
- Sigaudo-Roussel D, Demoit C, Fromy B, Koitka A, Leftheroitis G, Abraham P, Saumet JL. Early endothelial dysfunction severely impairs skin blood flow repsonse to local pressure application in streptozotocin-induced diabetic mice. Diabetes. 2004;53:1564-1569.
- Soriano FG, Virag L, Jactap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KGK, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly (ADP-ribose) polymerase activation. Nat Med. 2001;7:108-113.
- Stevens MJ, Dananberg J, Feldman EL, Lattimer SA, Kamijo M, Thomas TP, Shindo H, Sima AA, Greene DA. The linked roles of nitric oxide, aldose reductase, and (Na+, K+)-ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J Clin Invest. 1994;94:853-859.
- Stransberry KB, Peppard HR, Babyak LM, Popp G, McNitt PM, Vinik AI. Primary nociceptive afferents mediate the blood flow dysfunction in non-glaborous (hairy) skin of type 2 diabetes: a new model for the pathogenesis of microvascular dysfunction. Diabetes Care 1999;22:1549-1554.
- Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007
Dec;293(6):L1444-53 - Swiecki C, Stojadinovic A, Anderson J, Zhao A, Dawson H, Shea-Donohue T. Effect of hyperglycemia and nitric oxide synthase inhibition on heat tolerance and induction of heat shock protein 72 kDa in vivo. Am Surg. 2003 Jul;69(7):587-92
- Tarif N, Bakris G. The place for calcium channel blockers in the treatment of hypertension in patients with diabetes mellitus. Saudi J Kidney Dis Transpl. 2000 Oct-Dec;11(4):531-6.
- Verma S and Anderson TL. Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002;105:546-549.
- Watanabe H, Vriens J, Suh HS, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 2002;277(49):47044-47051.
- Webb P. Temperatures of skin, sub-cutaneous tissue, muscle and core in resting men in cold, comfortable, and hot conditions. Eur J Appl Physiol Occup Physiol. 1992;64:471-476.
- Weigert AL, Martin PY, Niederberger M, Higa EM, McMurtry IF, Gines P, Schrier RW. Endothelium-dependent vascular hyporesponsiveness without detection of nitric oxide synthase induction in aortas of cirrhotic rats. Hepatology. 1995 Dec:22(6):1856-65.
- Wissler EH. Pennes’ 1948 paper revisited. J Appl Physiol. 1998;85(1):35-41.


