T. Wadayama *, M. Oishi, A. Hatta
Department of Materials Science, Graduate School of Engineering, Tohoku University, Aobayama 6-6-02, Sendai 980-8579, Japan
Abstract
Surface enhanced Raman scattering (SERS) from samples prepared by spreading para-nitrobenzoic acid (PNBA) and adenosine powders over silver thin films was achieved. The SERS intensities of the ionized PNBA on the silver film increase with increased applied pressure through a cover-glass and reach a maximum at 0.6 MPa. In contrast, the signals caused by adenosine remain nearly unchanged under applied pressures of 0– 0.6 MPa. Beyond 0.6 MPa, the signals attributable to samples decrease in intensity. Atomic force microprobe images reveal that nanometer-scale surface morphology is changed by 0.8 MPa pressure, suggesting that the decrease in SERS intensity is related to pressure-induced morphological changes. Results obtained in this study indicate that SERS spectra are obtainable easily, without solvents, under ambient conditions using dispersion of the sample powder.
1. Introduction
A central problem in noble metal island films involves their interaction with electromagnetic waves. For example, interaction of nanometer-scale silver metal particles with visible light gives rise to distinct absorption because of the excitation of collective electron resonance. In addition, accompanying that electron resonance is an increase in electric field strength near the metal surface. The strengthened surface electric field considerably enhances (ca. 106) Raman scattered light of adsorbed molecules on the particles. This phenomenon is known as surface enhanced Raman scattering (SERS); it provides an effective surface probe for exploring metal– adsorbate interaction [1–3]. For that reason, SERS is powerful for obtaining information about molecular vibrational properties of adsorbates and is therefore applicable to investigation of various surface phenomena of noble metals. However, preparation methods of samples for SERS measurements are rather limited. For example, para-nitrobenzoic acid (PNBA) is often used for investigations of SERS to determine a new optical layout for exciting SERS or elucidating SERS mechanisms [4–8]. In published papers, PNBA was adsorbed on the SERS active substrate by dipping the substrate into the PNBA solution [4] or by spin-coating from the solution [5–8]. However, if sample molecules are dissolved only slightly into solvents, the above-mentioned “wet” processes are inapplicable. Alternatively, vacuum evaporation of analytes can be employed to prepare the samples. However, a vacuum chamber for sample evaporation should be necessary and thermal decomposition of the sample is more or less unavoidable. Therefore, a simple, “dry” process that is suitable for sample preparation of SERS is crucial, particularly for less-soluble or unstable analytes.
In this study, we propose a method for preparation of samples that are applicable to SERS measurements: the ‘‘pressure-attachment’’ (PA) method. In this method, analyte powders are spread over SERS active substrate. Consequently, analyte molecules are intact and no solvent is necessary. Furthermore, with increasing pressure, the amount of the sample that is directly attached to the SERS active substrate might increase, thereby improving spectral sensitivity.We have conducted Raman measurements for PNBA and adenosine sample powders spread over vacuum-evaporated silver thin films. Further, Raman intensity changes of the samples that occur by applying pressures through a cover-glass are investigated. We have observed SERS spectra of samples prepared using the PA method. The SERS intensities of ionized PNBA on the silver films increased with applied pressure and reach a maximum intensity at pressure of 0.6 MPa, whereas signals caused by adenosine remained nearly unchanged under pressures of 0.3–0.6 MPa. At 0.8 MPa, SERS signal intensities of both samples decreased. Atomic force microprobe (AFM) images show that applying 0.8 MPa pressure engenders nanometer-scale morphological changes of the vacuumevaporated silver surface. Results obtained in this study are presented herein.
2. Experimental
The silver films were deposited using vacuum evaporation of the metal with a resistively heated tungsten basket in the evaporation chamber (base pressure 3 x 10-9 Torr) on Pyrex glass (35 mm x 25 mm x 1 mm) substrates at room temperature. The pressure during evaporation was 2–5 x 10-8 Torr and the mass thickness of the silver thin film was monitored using a quartz oscillator. Although silver films that were about 5 nm in thickness gave the most intense Raman signals from adsorbed PNBA, 20 nm-thick silver films on the substrates were used in this study. Commercially purchased para-nitrobenzoic acid (PNBA 99% purity;Wako Pure Chemicals Industries, Ltd.) and adenosine (98% purity; Wako Pure Chemicals Industries, Ltd.) were used without further purification. After the silver vacuum evaporation on the substrate, SERS samples were prepared using the PA method, in which either PNBA or adenosine powder (ca. 15 mg) was dispersed directly onto the films using a spatula. Then a cover-glass (the same size as that for the glass substrate) was closely overlapped with the substrate; the excess PNBA powder was blown away using an air-blower after removing the cover-glass. Again, the cover-glass plate was pressed heading for the sample surface (spread molecules/Ag films) and Raman spectral measurements were carried out for the samples thus prepared. For comparison, the SERS sample of adsorbed PNBA on the silver films was formed through dipping of the substrate into an ethanol solution.
Fig. 1 shows a sample layout for our SERS measurements. Raman spectral measurements were conducted with and without applying pressures using a screw. The applied pressure was estimated using a pressure measurement film (prescale; Fuji Photo Film Co., Ltd.) that was placed between the coverglass plate and the Teflon sheet. The Raman spectrometer used in this study has been described elsewhere [9,10]. A He-Ne laser (633 nm; 10 mW at the sample position) was used as a radiation source for Raman excitations. The Raman scattered light from the sample was dispersed using a polychrometer (CT25TP; Jasco Inc.) and detected with a liquid-N2-cooled CCD detector (ST130; Princeton Instruments Inc.). The detection system was interfaced to a personal computer for data acquisition and storage. Surface morphologies of samples were imaged using AFM (Nano-Scope E; Veeco Instruments) under ambient conditions.
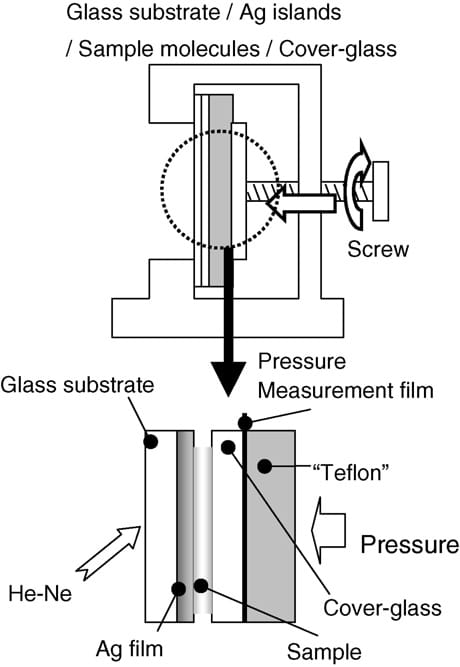
Fig. 1. Sample layout for SERS measurements.
3
. Results and discussion
Fig. 2 shows Raman spectra of bulk PNBA (a), PNBA powder spread over the silver (b), and PNBA adsorbed on the film by dipping the substrate into ethanol solution (c), in the region of 1550–1000 cm-1. During recording the spectrum of (b), no pressure was applied to the PNBA/silver substrate. The bands at 1348 and 1102 cm-1 on the spectrum of (a) are safely assigned to NO2 symmetric stretching and in-plane bending modes of PNBA, respectively [5–7]. Both bands also appeared on the spectra in the presence of the silver thin film (b) and (c), although PNBA prepared by the dipping method onto the bare glass substrate showed no Raman bands (not shown).
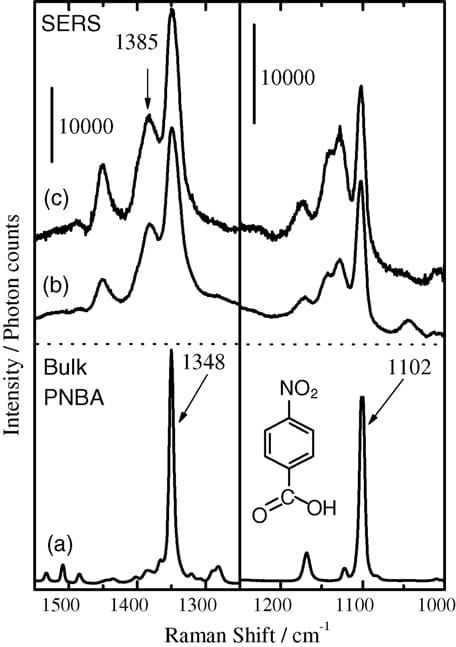
Fig. 2. SERS spectra of PNBA adsorbed on the silver surface. (a) bulk PNBA powder, (b) PNBA adsorbed on the silver by spreading PNBA powder, and (c) PNBA adsorbed on the silver through dipping the substrate into ethanol solution.
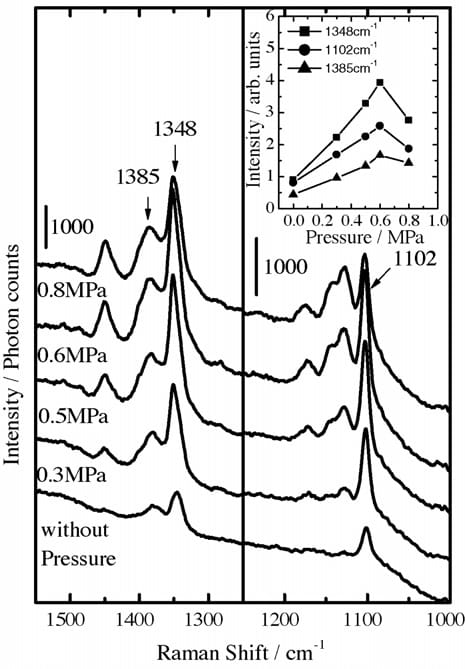
Fig. 3. SERS spectra of PNBA on the Ag thin film formed by the PA method as a function of applied pressures. Inset: Peak intensities vs. applied pressures.
The most noticeable feature of spectrum (c) is the appearance of the 1385 cm-1 band, which is ascribable to the COO- symmetric stretching mode. PNBA is well known to ionize on silver island films [4–8]. The COO- symmetric stretching band of the ionized PNBA is also located at 1385 cm-1 in the spectrum (b), indicating that PNBA ionization took place even through dispersion of PNBA powder over the silver film. At any rate, spreading sample powder over the vacuum-evaporated silver thin film engenders the SERS from the sample, revealing that SERS spectra are obtainable without using solvents
The SERS spectra from adsorbed PNBA ion as a function of applied pressure (0–0.8 MPa) are shown in Fig. 3. Intensities of the bands at 1385, 1348, and 1102 cm-1 versus the pressures are plotted in the inset. Although intense Raman signals are located at 1450 and 1130 cm-1 in Fig. 3, the two bands’ intensities depend not only on the pressure but also on the laser irradiation. The bands can be ascribed to photo-induced reduction products of PNBA [4,6,8]. Therefore, we exclude the intensity changes of bands from consideration.
As shown in Fig. 3, the three bands at 1385, 1348, and 1102 cm-1 increased in intensity with applied pressure up to 0.6 MPa. The 20 nm-thick silver thin film used as a SERS active substrate comprised several-tenths-of-nanometer-scale islands. In the initial stage of the PA process (merely spreading PNBA powder over the silver thin film), PNBA molecules might only slightly adsorb on the silver atoms located between islands at which a rather strong surface electric field should be generated through the collective electron resonances [1–3]. When applying pressures up to 0.6 MPa, free PNBA molecules probably migrate to the specific silver atoms: the SERS active site. High chemical affinity of PNBA molecules to Ag atoms engenders ionization of PNBA molecules on the SERS active sites, increasing to the bands’ intensity. This increase is reflected in the model of Fig. 4(a). In contrast, the application of 0.8 MPa pressure results in intensity reduction of the bands caused by ionized PNBA.
Fig. 5 shows SERS spectral changes of adenosine spread over the silver thin film with increasing pressure, along with the spectrum of bulk adenosine.We specifically examine the 1453, 1350, and 730 cm-1 bands that can be ascribed, respectively, to combinations of C–C stretching and C–H bending, C–N stretching, and ring breathing modes [11–15]. As shown in Fig. 5, the three bands on the spectra shift to higher frequencies than those on the spectrum of the bulk sample, indicating that a SERS signal from the adsorbed adenosine is obtainable.
Although SERS intensities of the bands caused by ionized PNBA increase with increasing pressure up to 0.6 MPa, the SERS spectra of adsorbed adenosine are insensitive to pressure (Fig. 5). In general, nucleoside molecules are held together in the crystal by a very comprehensive system of hydrogen bonds [16,17]. Therefore, to chemisorb an isolated adenosine molecule onto the SERS sites, excess energy should be necessary for breaking hydrogen bonds between adenosine molecules in the crystal (Fig. 4(b)). Accordingly, the SERS signals attributable to the adsorbed adenosine remained nearly unchanged by applying pressures of 0.3–0.6 MPa.
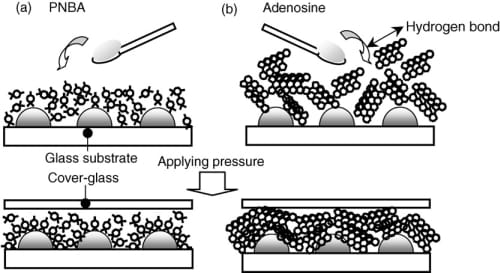
Fig. 4. Illustration of sample molecules spread over the Ag-island thin films: (a) PNBA and (b) adenosine.
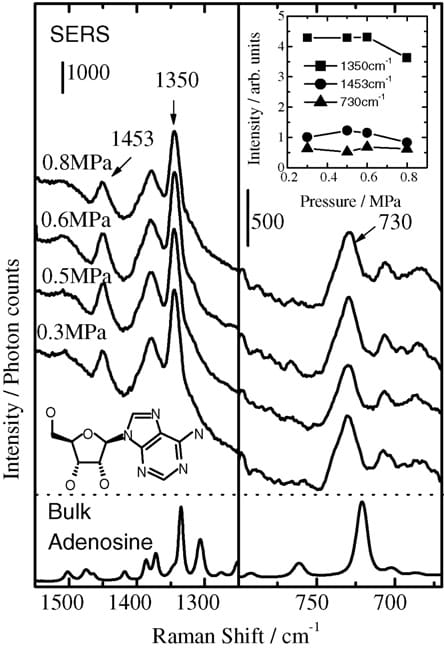
Fig. 5. SERS spectra of adenosine on the silver thin film formed using the PA method as a function of applied pressure. Inset: Peak intensities vs. applied pressures.
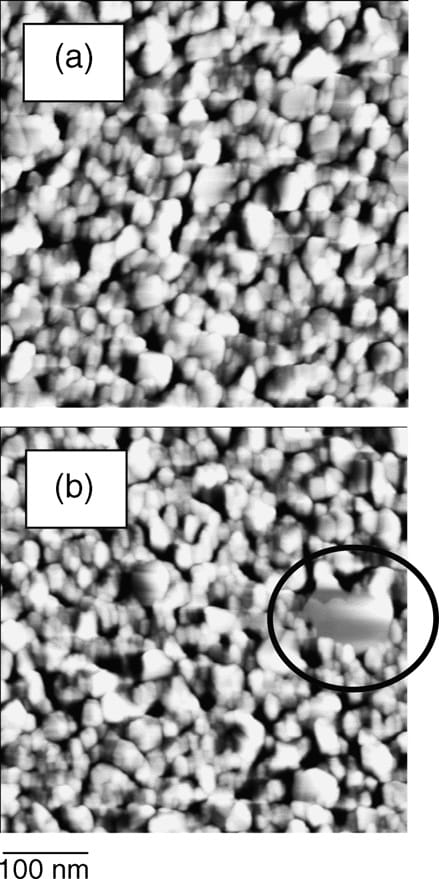
Fig. 6. AFM images before (a) and after (b) applying 0.8 MPa pressure.
The SERS intensities caused by adsorbed species decrease at 0.8 MPa pressure, irrespective of the sample (Figs. 3 and 5). Reduction of the band intensities might relate to the pressureinduced morphological change of the silver islands. For that reason, the silver surfaces can be inspected using AFM. Fig. 6 shows results of AFM inspections conducted before (a) and after (b) applying 0.8 MPa pressure. A rather flat area, marked with a circle, is visible in image (b). The SERS intensities caused by adsorbed molecules are well known to be quite sensitive for visualizing nanometer-scale morphology of noble metal islands. Actually, well-dispersed isolated noble metal colloidal spheres whose sizes are several-tenths of a nanometer show a maximum SERS enhancement factor for molecules adsorbed on the spheres [18,19]. The pressure-induced morphological change of the silver islands might reduce the SERS intensity of the bands caused by adsorbed species, but the specific nanometer-scale structure responsible for SERSactivation has not been clarified.
4. Summary
Raman spectral measurements were conducted for PNBA and adenosine powders spread over silver thin films. The Raman signals caused by adsorbed PNBA ion and adenosine were obtained, showing that the PA method is useful for preparing SERS samples without using solvents. The SERS intensities of the ionized PNBA on the silver film increased with increasing pressure and reached a maximum at 0.6 MPa, whereas those of adenosine remained nearly unchanged. The 0.8 MPa pressure engenders SERS intensity reduction in both samples. Our AFM inspections of the silver film surfaces suggest that the decreased SERS intensities are related to pressure-induced changes in silver surface morphology. Results obtained in this study indicate that SERS spectra can be obtained easily using dispersion of the sample powder on the silver film.
References
- R.K. Chang, T.E. Furtak (Eds.), Surface Enhanced Raman Scattering, Plenum, New York, 1982.
- A. Otto, Light Scattering in Solids IV, in: M. Cardona, G. Gu¨ntherodt (Eds.), Electronic Scattering, Spin Effects, SERS, and Morphic Effects, Springer, Berlin, 1983, p. 289.
- A. Campion, J.T. Yates, Jr., T.E. Madey (Eds.), Vibrational Spectroscopy of Molecules on Surfaces, Plenum, New York, 1987.
- S. Sun, R.L. Brike, J.R. Lombardi, K.P. Leung,
A.Z. Genack, J. Phys. Chem. 92 (1988) 5965. - H. Bercegol, F.J. Boerio, J. Phys. Chem. 99 (1995) 8763.
- P.G. Roth, R.S. Venkatachalam, F.J. Boerio, J. Chem. Phys. 85 (1986) 1150.
- H. Bercegol, F.J. Boerio, Langmuir 10 (1994) 3684.
- R.S. Venkatachalam, F.J. Boerio, P.G. Roth, J. Raman Spectrosc. 19 (1988) 281.
- T. Wadayama, S. Yamamoto, H. Hatta, Appl. Phys. Lett. 65 (1994) 1653.
- T. Wadayama, T. Arigane, A. Hatta, Appl. Phys. Lett. 73 (1998) 2570.
- B. Giese, D. McNaughton, J. Phys. Chem. B106 (2002) 101.
- C. Otto, T.J.J. van der Tweel, F.M.M. de Mul, J. Greve, J. Raman Spectrosc. 17 (1986) 289.
- C. Li, J. Huang, Y. Liang, Spectrochim. Acta A57 (2001) 1587.
- T. Theophanides, S. Hanessian, M. Manfait, M. Berjot, J. Raman Spectrosc. 16 (1985) 32.
- Z.H. Xu, I.S. Butler, K.K. Ogilvie, J. Raman Spectrosc. 28 (1997) 901.
- S. Furberg, Acta Cryst. 3 (1950) 325.
- S. Furberg, C.S. Petersen, C. Rømming, Acta Cryst. 18 (1965) 313.
- R.G. Freeman, K.C. Grabar, K.J. Allison, R.M. Bright, J.A. Davis, A.P. Guthrie, M.B. Hommer, M.A. Jackson, P.C. Smith, D.G. Walter, M.J. Natan, Science 267 (1995) 1629.
- K. Kneipp, H. Kneipp, R. Manoharan, E.B. Hanlon, I. Itzkan, R.R. Dasari, M.S. Feld, Appl. Spectrosc. 52 (1998) 1493.


