Noritoshi Nagaya, MD; Masaaki Uematsu, MD; Masayasu Kojima, MD; Yoshihiko Ikeda, MD;
Fumiki Yoshihara, MD; Wataru Shimizu, MD; Hiroshi Hosoda, MD; Yuki Hirota, PhD;
Hideyuki Ishida, MD; Hidezo Mori, MD; Kenji Kangawa, PhD
Background — Ghrelin is a novel growth hormone (GH)–releasing peptide that may also induce vasodilation and stimulate feeding through GH-independent mechanisms. We investigated whether ghrelin improves left ventricular (LV) dysfunction and attenuates cardiac cachexia in rats with chronic heart failure (CHF). Methods and Results — Ligation of the left coronary artery or sham operation was performed; 4 weeks after surgery, rat ghrelin (100 mg/kg SC BID) or saline was administered for 3 weeks. Echocardiography and cardiac catheterization were performed. Serum GH and insulin-like growth factor-1 were significantly higher in both CHF and sham rats treated with ghrelin than in those given placebo (P<0.05 for both). CHF rats given placebo showed an impaired increase in body weight compared with sham rats given placebo (P<0.05). CHF rats treated with ghrelin, however, showed a significantly greater increase in body weight than those given placebo (110% versus 13%, P<0.05). They showed significantly higher cardiac output (315±49 versus 266±31 mL · min21 · kg21, P<0.05) and LV dP/dtmax (5738±908 versus 4363±973 mm Hg/s, P<0.05) than CHF rats given placebo. Ghrelin increased diastolic thickness of the noninfarcted posterior wall, inhibited LV enlargement, and increased LV fractional shortening in CHF rats (from 1563% to 1963%, P,0.05). Conclusions — Chronic subcutaneous administration of ghrelin improved LV dysfunction and attenuated the development of LV remodeling and cardiac cachexia in rats with CHF. (Circulation. 2001;104:1430-1435.)
Left ventricular (LV) remodeling (dilatation and wall thinning) and cardiac cachexia (body weight loss and muscle wasting) are often observed in patients with end-stage chronic heart failure (CHF).1,2 Growth hormone (GH) and its mediator, insulin-like growth factor-1 (IGF-1), are essential for skeletal and myocardial growth and metabolic homeostasis.3,4 Earlier studies have shown that GH supplementation may have beneficial effects on myocardial structure and function in some patients with CHF,5–7 although neutral results of randomized trials have also been reported.8,9 Elevated serum GH with normal or low IGF-1 levels was observed in cachectic patients with CHF.10,11 These findings indicate that the GH/IGF-1 axis may be related to LV dysfunction and the resultant cachexia.
Ghrelin is a novel GH-releasing peptide, isolated from the stomach, that is identified as an endogenous ligand for GH secretagogue receptor (GHS-R).12 Considering the hemodynamic and anabolic effects of GH/IGF-1, ghrelin may have beneficial effects on LV function and energy metabolism in CHF through GH-dependent mechanisms. Conversely, ghrelin may have direct cardiovascular and metabolic effects through GH-independent mechanisms: GHS-R mRNA is detected not only in the hypothalamus and pituitary but also in the heart and vessels,13 stimulation of GHS-R has been shown to prevent cardiac damage after ischemia-reperfusion in hypophysectomized rats,14 intravenous injection of ghrelin decreases arterial pressure and increases cardiac output in healthy humans,13 and ghrelin causes a positive energy balance by stimulating food intake15 and inducing adiposity.16 These findings raise the possibility that administration of ghrelin may be beneficial in cachectic CHF.
Thus, the purposes of this study were (1) to investigate whether ghrelin supplementation improves LV myocardial structure and function in rats with CHF and (2) to examine whether ghrelin attenuates the development of cardiac cachexia.
Methods
Model of CHF
Myocardial infarction was produced in male Wistar rats weighing 200 to 240 g by left coronary ligation as described previously.17 The control rats underwent a sham operation consisting of thoracotomy and cardiac exposure but without coronary artery ligation. The surviving rats were maintained on standard rat chow. There was a 33% mortality rate within 48 hours after coronary ligation, and the subsequent mortality was 15% for 4 weeks. Four weeks after surgery, 31 infarct rats were randomly divided into 2 groups and given either ghrelin (n=16) or placebo (n=15) for 3 weeks. Similarly, 26 sham-operated rats were randomized to receive ghrelin (n=13) or placebo (n=13).
Administration of Ghrelin
Rat ghrelin was obtained from the Peptide Institute Inc. Four weeks after surgery, rat ghrelin (100 μg/kg BID for 3 weeks) or saline was injected subcutaneously in both CHF rats and sham-operated rats.
Echocardiographic Studies
Echocardiographic studies were performed before and after 3-week supplementation with ghrelin. 2D targeted M-mode tracings were obtained at the level of the papillary muscles. Anterior and posterior end-diastolic and end-systolic wall thickness, LV end-diastolic and end-systolic dimensions, and LV fractional shortening were measured by the American Society for Echocardiology leading-edge method from 3 consecutive cardiac cycles. LV meridional wall stress was estimated as 0.334 x LV pressure x [LV dimension/(11PWT/LV dimension)], where PWT is posterior wall thickness.18 LV pressure was recorded within 12 hours of the final echocardiogram.
Hemodynamic Studies
Hemodynamic studies were performed after 3 weeks of treatment with ghrelin or placebo. After anesthesia with pentobarbital, polyethylene catheters were inserted into the right femoral artery and the right ventricle (RV). Hemodynamic variables were measured with a pressure transducer connected to a polygraph. A 1.5F micromanometer- tipped catheter was advanced into the LV and then replaced with a thermomicroprobe for measurement of cardiac output.
After completion of these measurements, blood was drawn from the femoral artery for measurements of GH and IGF-1. Infarct size was determined as a percentage of LV area, as reported previously.17 Only rats with infarct size >25% of the LV were enrolled in this study as a model of CHF. The right gastrocnemius muscle was weighed, and its protein content was determined by the Lowry method to evaluate the ratios of muscle to tibial length and muscle to body weight in 4 groups (n=5 each).
Histological Examination
LV myocardium was fixed in 10% formalin. Three cross sections in noninfarcted myocardium, cut from apex to base, were obtained from individual rats for comparison among 4 groups (n=5 each). They w
ere embedded in paraffin and stained with hematoxylin-eosin for assessment of myocardial hypertrophy and with Masson’s trichrome for measurement of interstitial fibrosis. Muscle fiber diameter was evaluated by direct measurements at x400 magnification only in cross sections that included a nuclear profile.19 A total of 40 cells per section were evaluated. After 3 sections per animal and 20 fields per section were scanned and computerized with a digital image analyzer (Win Roof, Mitani Co), volume collagen fraction was calculated as the sum of all connective tissue areas divided by the total area of the image.
Hormone Assays
Serum GH was measured with a radioimmunoassay kit (Biotrak RIA, Amersham). Serum IGF-1 was determined with an enzyme immunoassay kit (Active Rat IGF-1 EIA, DSL Inc).
Acute Hemodynamic Effects of Ghrelin
Acute hemodynamic responses to intravenous injection of ghrelin (10 μg/kg) or placebo were examined in sham rats, CHF rats, and spontaneous dwarf rats, a GH-deficient rat model that carries a disrupted GH gene20 (n=5 each).
To investigate whether ghrelin has direct inotropic effects on cardiac myocytes, adult cardiac myocytes (n56) were isolated from the LV of normal rats by collagenase digestion.21 Cardiac myocytes were also isolated from the LV noninfarcted region of CHF rats that received 3-week treatment with ghrelin (n=40) or placebo (n=38) to examine the chronic effects of ghrelin treatment on myocyte contractile function. Myocyte contraction was monitored every 4 ms by a high-speed CCD camera (Fastcam-net, Photoron) and measured with NIH Image software (version 1.62, NIH). Myocytes were stimulated at 0.5 Hz with 7-ms pulses.
Numerical values were expressed as mean±SD. Comparisons of parameters among the 4 groups were made with 1-way ANOVA, followed by the Newman-Keuls test. Changes in parameters during treatment were analyzed with 2-way ANOVA for repeated measures, followed by the Newman-Keuls test. The effects of ghrelin on myocyte motion were analyzed by paired Student’s t test. Comparisons of parameters between 2 groups were made by unpaired Student’s t test. A value of P<0.05 was considered significant.
Results
Effects of Ghrelin on Somatotropic Function and Body Weight
Moderate to large infarcts were observed in both CHF groups, and the mean infarct size was similar in the two (Table 1). Serum GH and IGF-1 were significantly higher in both CHF and sham rats treated with ghrelin than in those given placebo (Figure 1). CHF rats given placebo showed an impaired increase in body weight compared with sham rats given placebo (Figure 2). CHF rats treated with ghrelin, however, showed a significantly greater increase in body weight than those given placebo. Tibial length was significantly increased in rats treated with ghrelin compared with those given placebo (Table 1). LV weight/tibial length was significantly higher in sham rats treated with ghrelin than in those given placebo. RV weight/tibial length was significantly lower in CHF rats treated with ghrelin than in those given placebo. The ratios of gastrocnemius muscle weight to tibial length and muscle protein content to tibial length were significantly lower in CHF rats given placebo than in sham rats given placebo, suggesting the presence of cachexia in this model of CHF. Both parameters, however, were significantly higher in CHF rats treated with ghrelin than in those given placebo.
Effects of Ghrelin on Hemodynamics
Heart rate, mean arterial pressure, RV systolic pressure, and mean right atrial pressure tended to be lower in CHF rats treated with ghrelin than those given placebo, although these changes did not reach statistical significance (Table 2). Cardiac output, stroke volume, and LV dP/dtmax were significantly higher in CHF rats treated with ghrelin than in those given placebo (Figure 3), whereas LV end-diastolic pressure and LV dP/dtmin were significantly lower in CHF rats given ghrelin (Table 2). Systemic vascular resistance was significantly lower in CHF rats treated with ghrelin than in those given placebo (Figure 3).
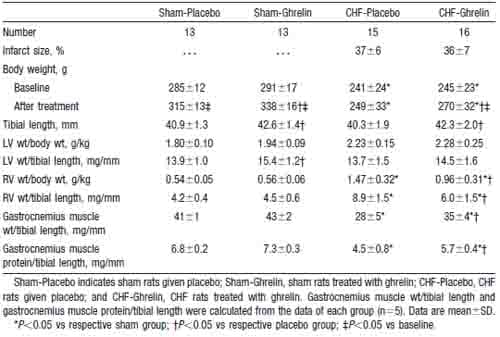
TABLE 1. Characterization of the Animals
Effects of Ghrelin on LV Geometry and Function
Ghrelin significantly increased diastolic thickness of the noninfarcted posterior wall in both CHF and sham rats (Figure 4). LV diastolic dimension tended to decrease in CHF rats treated with ghrelin, although it tended to increase in those given placebo. Thus, LV diastolic dimension was significantly smaller in CHF rats treated with ghrelin than in those given placebo. As a result, LV wall stresses in systole and diastole were significantly lower in CHF rats treated with ghrelin than those given placebo (Table 3). LV fractional shortening increased significantly in CHF rats treated with ghrelin, although it tended to decrease in CHF rats given placebo (Figure 4).
Histological Analysis
Muscle fiber diameter of the noninfarcted myocardium was significantly larger in CHF rats treated with ghrelin than those given placebo (Figure 5). There was no significant difference in collagen volume fraction between CHF rats treated with ghrelin and those given placebo.
Acute Hemodynamic Response to Ghrelin
A single injection of ghrelin significantly decreased mean arterial pressure in both sham-operated rats (-8 mm Hg, P<0.05) and CHF rats (-7 mm Hg, P<0.05). The hypotensive effect was also observed in GH-deficient rats (-6 mm Hg, P<0.05). Ghrelin significantly decreased systemic vascular resistance in sham, CHF, and GH-deficient rats (212%, 213%, and 210%, P<0.05, respectively). There were no significant changes in heart rate, LV end-diastolic pressure, or LV dP/dtmax in each group (data not shown). Placebo injection had no effect on these hemodynamic parameters (data not shown).
Isolated Myocyte Contractile Function
Fractional cell shortening relative to baseline value was not significantly altered by each dose of ghrelin (9963% for 1 pmol/mL, 97617% for 10 pmol/mL, and 91615% for 100 pmol/mL), suggesting that ghrelin has no direct inotropic effects. In contrast, fractional cell shortening in myocytes of CHF rats treated with ghrelin for 3 weeks was significantly greater than that in CHF rats given placebo (1961% versus 1761%, P,0.05). Shortening velocity in ghrelin-treated CHF rats was significantly increased compared with that in CHF rats given placebo (278±14 versus 213±10 mm/s, P<0.001).
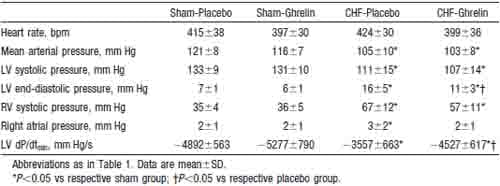
TABLE 2. Effects of Ghrelin on Hemodynamic Parameters
Discussion
Ghrelin is a novel GH-releasing peptide, isolated from the stomach, that acts through a mechanism independent of that of hypothalamic GH-releasing hormone.12 The GH-releasing effects of ghrelin are thought to be mediated by specific receptors, GHS-R, present mainly in the pituitary. Taken together with our results that administration of ghrelin increased serum GH and IGF-1 in both CHF and sham rats, this molecule may reach and act on the anterior pituitary via the blood circulation.
In the present study, chronic treatment with ghrelin tended to decrease mean arterial pressure and significantly decreased systemic vascular resistance in CHF rats. IGF-1 has been shown to cause vasodilation through a stimulatory effect on nitric oxide synthesis,22 suggesting that the vasodilator effects of ghrelin may be mediated at least in part via IGF-1. Conversely, we recently found that GHS-R exists in blood
vessels and that a single injection of ghrelin induces a hypotensive effect without an increase in IGF-1.13 In the present study, a decrease in systemic vascular resistance by ghrelin injection was observed not only in CHF rats but also in GH-deficient rats. These findings indicate that ghrelin has GH/IGF-1–independent vasodilatory effects.
This study also demonstrated that long-term administration of ghrelin improved cardiac performance in CHF rats, as indicated by increases in cardiac output, stroke volume, LV dP/dtmax, and LV fractional shortening and by increases in fractional cell shortening and shortening velocity of isolated myocytes. Although the GHS-R gene has been shown to be abundantly expressed in the myocardium,13 the present study could not demonstrate direct inotropic effects of ghrelin on isolated cardiac myocytes. Thus, a decrease in systemic vascular resistance by ghrelin may be responsible for the increased cardiac output. Alternatively, because GH upregulates sarcoplasmic Ca21-ATP and thereby enhances myocardial contractility,23 part of the cardiac effects of ghrelin may be mediated by GH. In the present study, treatment with ghrelin increased posterior wall thickness, inhibited the progressive LV enlargement, and thereby reduced the LV wall stress in rats with CHF. Histological analysis also demonstrated that ghrelin induced a cardiac hypertrophic response without development of significant fibrosis. GH and IGF-1 have been shown to enhance physiological compensatory hypertrophy in rats after myocardial infarction, resulting in a decrease in LV wall stress leading to improvement in cardiac function.24 Thus, ghrelin may also improve cardiac function through GH/IGF-1–dependent mechanisms. Taken together, an improvement in cardiac function by ghrelin may be related not only to its effects on the GH/IGF-1 axis and but also to a result of its vasodilatory effects.
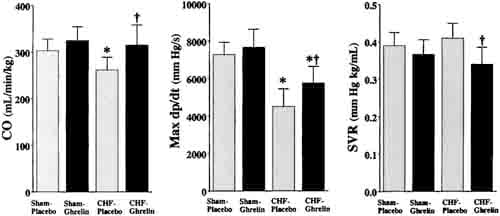
Figure 3. Effects of ghrelin on hemodynamic parameters in CHF and sham rats. CO indicates cardiac output; SVR, systemic vascular resistance. Data are mean6SD. *P < 0.05 vs sham group; †P,0.05 vs placebo group.
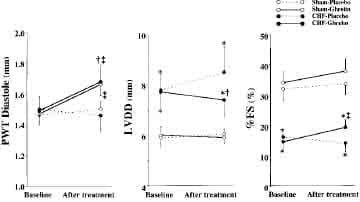
Figure 4. Effects of ghrelin on LV geometry and function determined by echocardiography in CHF and sham rats. PWT indicates posterior wall thickness; LVDD, LV diastolic dimension; and %FS, LV fractional shortening. Data are mean ±SD. *P,0.05 vs sham group; †P < 0.05 vs placebo group; ‡P < 0.05 vs baseline.
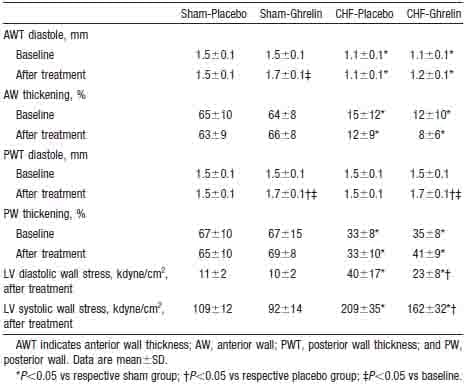
TABLE 3. Echocardiographic Data
Cardiac cachexia, which is a catabolic state characterized by weight loss and muscle wasting, occurs frequently in patients with end-stage CHF2,25–27 and is a strong independent risk factor for mortality in patients with CHF.27 In the present study, CHF rats given placebo showed an impaired increase in body weight and a significant decrease in the muscle/bone ratio, suggesting the presence of cardiac cachexia. In contrast, CHF rats treated with ghrelin revealed an appropriate increase in body weight and a preserved muscle-to-bone ratio similar to those observed in sham-operated rats. The ratios of gastrocnemius muscle weight to body weight and of protein content to body weight were significantly higher in CHF rats treated with ghrelin than in those given placebo (0.53±0.04% versus 0.46±0.07% and 0.09±0.01% versus 0.07±0.01%, both P<0.05), and mean right atrial pressure did not increase significantly in those given ghrelin. These data indicate that the increase in body weight by ghrelin was not due to peripheral fluid retention but rather to muscle weight gain. Tschop et al16 showed that administration of ghrelin induces a positive energy balance and weight gain by decreasing fat utilization and increasing carbohydrate utilization through a GH-independent mechanism. In addition, ghrelin has been shown to elicit a potent, long-lasting stimulation of food intake via activation of neuropeptide Y neurones in the hypothalamic arcuate nucleus.15 Furthermore, ghrelin enhanced production of GH and IGF-1, both of which cause anabolic effects such as maintenance of skeletal muscle mass and myocardial growth.3,4 These findings suggest that ghrelin may play an important role in the regulation of metabolic balance and in preventing cardiac cachexia through GHdependent or -independent effects.
Although many animal studies have documented beneficial effects of GH,5,6,24 controlled studies in humans have been predominantly negative.8,9 Nevertheless, ghrelin may have additional therapeutic potential because it has GHindependent effects, such as vasodilatory actions and anticachectic effects.
Conclusions
Chronic subcutaneous administration of ghrelin improved LV dysfunction and attenuated the development of LV remodeling and cardiac cachexia in rats with CHF. Administration of ghrelin may be a new therapeutic approach to the treatment of CHF.
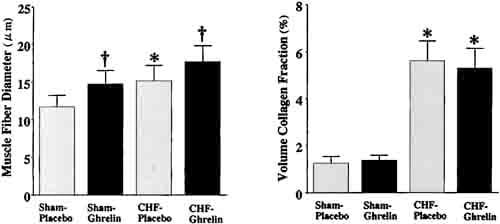
Figure 5. Effects of ghrelin on myocyte growth and collagen density in CHF and sham rats. Data are mean ±SD. *P < 0.05 vs sham group; †P,0.05 vs placebo group.
Acknowledgments
This work was supported in part by a Research Grant for Cardiovascular Disease (12C-2) from the Ministry of Health, Labor, and Welfare; the Uehara Memorial Foundation; JSPS-RFIF 97I 00 201, Science Frontier program of MECSST, NEDO; and the Promotion of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research (OPSR) of Japan.
References
- Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564 –1575.
- Pittman JG, Cohen P. The pathogenesis of cardiac cachexia. N Engl J Med. 1964;271:403– 409.
- Amato G, Carella C, Fazio S, et al. Body composition, bone metabolism, heart structure and function in growth hormone deficient adults before and after growth hormone replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77:1671–1676.
- Fuller J, Mynett JR, Sugden PH. Stimulation of cardiac protein synthesis by insulin-like growth factors. Biochem J. 1992;282:85–90.
- Yang R, Bunting S, Gillett N, et al. Growth hormone improves cardiac performance in experimental heart failure. Circulation. 1995;92:262–267.
- Cittadini A, Stromer H, Katz SE, et al. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat: a combined in vivo and in vitro evaluation. Circulation. 1996;93:800–809.
- Fazio S, Sabatini D, Capaldo B, et al. A preliminary study of growth hormone in the treatment of dilated cardiomyopathy. N Engl J Med. 1996;334:809–814.
- Osterziel KJ, Strohm O, Schuler J, et al. Randomised, double-blind, placebo-controlled trial of human recombinant growth hormone in patients with chronic heart failure due to dilated cardiomyopathy. Lancet. 1998;351:1233–1237.
- Isgaard J, Bergh CH, Caidahl K, et al. A placebo-controlled study of growth hormone in patients with congestive heart failure. Eur Heart J. 1998;19:1704 –1711.
- Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/ anabolic imbalance in chronic heart failure and their importance in cardiac cachexia. Circulation. 1997;96:526 –534.
- Niebauer J, Pflaum CD, Clark AL, et a
l. Deficient insulin-like growth factor I in chronic heart failure predicts altered body composition, anabolic deficiency, cytokine and neurohormonal activation. J Am Coll Cardiol. 1998;32:393–397. - Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormonereleasing acylated peptide from stomach. Nature. 1999;402:656–660.
- Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol. 2001;280: R1483–R1487.
- Locatelli V, Rossoni G, Schweiger F, et al. Growth hormone-independent cardioprotective effects of hexarelin in the rat. Endocrinology. 1999;140: 4024–4031.
- Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194 –198.
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908 –913.
- Nagaya N, Nishikimi T, Yoshihara F, et al. Cardiac adrenomedullin gene expression and peptide accumulation after acute myocardial infarction in rats. Am J Physiol. 2000;278:R1019–R1026.
- Douglas PS, Reichek N, Plappert T, et al. Comparison of echocardiographic methods for assessment of LV shortening and wall stress. J Am Coll Cardiol. 1987;9:945–951.
- Douglas PS, Tallant B. Hypertrophy, fibrosis and diastolic dysfunction in early canine experimental hypertension. J Am Coll Cardiol. 1991;17: 530–536.
- Takeuchi T, Suzuki H, Sakurai S, et al. Molecular mechanism of growth hormone (GH) deficiency in the spontaneous dwarf rat: detection of abnormal splicing of GH messenger ribonucleic acid by the polymerase chain reaction. Endocrinology. 1990;126:31–38.
- Ishida H, Genka C, Hirota Y, et al. Formation of planar and spiral Ca21 waves in isolated cardiac myocytes. Biophys J. 1999;77:2114 –2122.
- Boger RH, Skamira C, Bode-Boger SM, et al. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency: a double-blind, placebo-controlled study. J Clin Invest. 1996;98:2706 –2713.
- Tajima M, Weinberg EO, Bartunek J, et al. Treatment with growth hormone enhances contractile reserve and intracellular calcium transients in myocytes from rats with postinfarction heart failure. Circulation. 1999;99:127–134.
- Cittadini A, Grossman JD, Napoli R, et al. Growth hormone attenuates early left ventricular remodeling and improves cardiac function in rats with large myocardial infarction. J Am Coll Cardiol. 1997;29: 1109–1116.
- Anker SD, Coats AJS. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115: 836–847.
- Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323: 236–241.
- Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349: 1050–1053.


